Key Points
A phase 1 trial of the anti-CD22 immunotoxin moxetumomab pasudotox was conducted in children with ALL.
A 32% objective response rate was observed, including 11 composite complete responses (23%), 5 of which were minimal residual disease negative.
Novel therapies are needed to overcome chemotherapy resistance for children with relapsed/refractory acute lymphoblastic leukemia (ALL). Moxetumomab pasudotox is a recombinant anti-CD22 immunotoxin. A multicenter phase 1 study was conducted to determine the maximum-tolerated cumulative dose (MTCD) and evaluate safety, activity, pharmacokinetics, and immunogenicity of moxetumomab pasudotox in children, adolescents, and young adults with ALL (N = 55). Moxetumomab pasudotox was administered as a 30-minute IV infusion at doses of 5 to 50 µg/kg every other day for 6 (cohorts A and B) or 10 (cohort C) doses in 21-day cycles. Cohorts B and C received dexamethasone prophylaxis against capillary leak syndrome (CLS). The most common treatment-related adverse events were reversible weight gain, hepatic transaminase elevation, and hypoalbuminemia. Dose-limiting CLS occurred in 2 of 4 patients receiving 30 µg/kg of moxetumomab pasudotox every other day for 6 doses. Incorporation of dexamethasone prevented further dose-limiting CLS. Six of 14 patients receiving 50 µg/kg of moxetumomab pasudotox for 10 doses developed hemolytic uremic syndrome (HUS), thrombotic microangiopathy (TMA), or HUS-like events, exceeding the MTCD. Treatment expansion at 40 µg/kg for 10 doses (n = 11) exceeded the MTCD because of 2 HUS/TMA/HUS-like events. Dose level 6B (ie, 50 µg/kg × 6 doses) was the MTCD, selected as the recommended phase 2 dose. Among 47 evaluable patients, an objective response rate of 32% was observed, including 11 (23%) composite complete responses, 5 of which were minimal residual disease negative by flow cytometry. Moxetumomab pasudotox showed a manageable safety profile and evidence of activity in relapsed or refractory childhood ALL. This trial was registered at www.clinicaltrials.gov as #NCT00659425.
Introduction
Despite substantial progress in the curative treatment of childhood acute lymphoblastic leukemia (ALL), the outlook is guarded for patients with high-risk features and those who relapse, and ALL remains a leading cause of cancer-related mortality in children.1,-3 Current therapies have considerable treatment-associated morbidity and mortality risks.4 New therapeutic approaches are needed to overcome chemotherapy resistance and reduce nonspecific toxicities.
Monoclonal antibody–based therapies have the potential to achieve these goals.5 CD22, a B-lymphoid differentiation antigen expressed on the surface of B-lineage ALL blasts,6 is a well-characterized therapeutic target for B-lineage ALL.7 On antigen binding, anti-CD22–directed agents are rapidly internalized by receptor-mediated endocytosis, making CD22 a particularly relevant target for immunotoxins.7,8 Previously, we constructed the recombinant immunotoxin RFB4(dsFv)-PE38 (BL22; CAT-3888) consisting of the variable domains of the murine anti-CD22 monoclonal antibody RFB4 fused to a 38-kDa fragment of Pseudomonas exotoxin A.9 The first-generation agent, BL22, had an acceptable safety profile but only modest activity in a pediatric phase 1 trial.10 A second-generation agent with higher affinity for CD22 (moxetumomab pasudotox [CAT-8015, HA22]) showed increased in vitro cytotoxicity against childhood ALL.11,12 Here, we report the results of a phase 1 clinical trial in which moxetumomab pasudotox displayed a manageable and acceptable safety profile and induced complete responses (CRs) in children, adolescents, and young adults with multiply relapsed or chemotherapy-refractory ALL.
Patients and methods
This trial was conducted at the National Institutes of Health Clinical Center (Bethesda, MD), St Jude Children’s Research Hospital (Memphis, TN), Dana-Farber Cancer Institute/Boston Children’s Hospital (Boston, MA), Hospital for Sick Children (Toronto, ON, Canada), and Children’s Hospital Los Angeles (Los Angeles, CA) in compliance with the Declaration of Helsinki. The protocol was approved by the investigational review boards at all sites. All patients or their legal guardians provided written informed consent.
Eligibility
Patients age 6 months to 25 years with multiply relapsed or chemotherapy-refractory ALL who had received ≥1 standard and 1 salvage regimen or allogeneic stem-cell transplant were eligible. Bone marrow involvement with ≥5% blasts was required, with blasts being CD22+ (ie, ≥30% of blasts expressing CD22 by flow cytometry). Patients could not receive chemotherapy within 14 days before first moxetumomab pasudotox dose, except for intrathecal or ALL maintenance chemotherapy. Individuals with isolated testicular relapse or active central nervous system involvement were excluded. Eligibility required aspartate aminotransferase and alanine aminotransferase ≤5 times the normal upper limit, total bilirubin ≤2 times the normal upper limit, and age-adjusted normal creatinine.
Flow cytometry and antigen-binding site determination
CD22 antigen expression was determined by flow cytometry at the National Cancer Institute. Antigen-site density was quantified by determining the anti-CD22 antibody–binding capacity per cell using the QuantiBRITE system for fluorescence quantitation (BD Biosciences, San Jose, CA) as previously described.13 Determination of minimal residual disease (MRD) in patients who achieved CR was performed by flow cytometry on bone marrow aspirate samples using standard methodology.14
Study design
The objectives were to estimate the maximum-tolerated cumulative dose (MTCD), defined as the highest dose and number of doses per cycle that could be administered safely (based on the safety profile); to describe the pharmacokinetics and immunogenicity; and to evaluate the antitumor activity of moxetumomab pasudotox in patients with relapsed/refractory ALL. Moxetumomab pasudotox 5 to 50 µg/kg was administered via IV infusion over 30 minutes every other day in a 21-day cycle (dose levels summarized in Table 3 in “Results”). During the trial, the treatment schedule was modified to increase the number of doses per cycle from 6 to 10 to reduce early progression or relapse by eliminating the treatment-free interval of the 6-dose cycle. The dose-limiting toxicity (DLT) period comprised the first 21-day cycle or lasted until resolution of toxicity to meet criteria for next treatment cycle initiation. Treatment cycles were repeated after ≥21 days in patients who did not have DLT (defined in “Toxicity grading and definition”) until progressive disease, grade 3/4 allergic events, or other reason for discontinuation. Dosing delays of ≤1 week for resolution of grade 2 toxicity or ≤2 weeks for scheduling conflict were permitted.
The initial cohort (cohort A) comprised an accelerated dose-escalation phase, with 1 patient each receiving 5, 10, and 20 µg/kg for 6 doses per cycle without dexamethasone. Subsequent cohorts (cohorts B and C) received prophylaxis against capillary leak syndrome (CLS) with dexamethasone 2.5 mg/m2 every 12 hours before and after the first 6 moxetumomab pasudotox doses during cycle 1. Cohort B comprised standard 3+3 escalation groups at 20, 30, 40, and 50 µg/kg for 6 doses per cycle. Cohort C used 10 doses per cycle. The final dose level (5C) used drug product developed using a new manufacturing process (ie, process 3),15 with greater bioactivity compared with the prior product (processes 1 and 2) based on in vitro cytotoxicity assays. Consequently, dose level 5C was adjusted to a dose of 32 µg/kg, 80% of the 40-µg/kg dose of prior drug product (hereafter referred to as 32 [40] µg/kg), to account for the difference in bioactivity and comprised 10 doses of moxetumomab pasudotox.
Supportive care
Hydration at a rate of ≥90 mL/m2 per hour occurred 3 hours before through 2 hours after each dose, with daily fluid intake approximating 1440 mL/m2 per day until 18 hours after the last dose of each cycle. Premedication with acetaminophen, diphenhydramine, and ranitidine was provided, as was additional standard supportive care as clinically indicated, such as antimicrobial therapy and tumor lysis syndrome prophylaxis.
Toxicity grading and definition
The National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) were used for toxicity and adverse event (AE) reporting.16 Dose-limiting toxicity was defined as nonhematologic toxicity deemed related to moxetumomab pasudotox of grade 2 lasting >28 days or at least grade 3, with the following exceptions: tumor lysis syndrome, abnormal electrolytes responding to supplementation, grade 3 hepatic dysfunction with resolution before the scheduled start of the next cycle, grade 4 transaminase elevation lasting <72 hours, grade 3 or 4 infection or neutropenic fever, or grade 3 fever, hypertriglyceridemia, hypercholesterolemia, or hypoalbuminemia in the absence of CLS. Hematologic DLT was defined as grade 4 hematologic toxicity lasting >5 days or for which transfusion was required. Patients with abnormal blood counts resulting from bone marrow infiltration were not evaluable for hematologic toxicity assessment. Hemolytic uremia syndrome (HUS) was graded as defined in supplemental Table 1, available on the Blood Web site.
Pharmacokinetics
Plasma levels of moxetumomab pasudotox were determined using a validated sandwich enzyme-linked immunosorbent assay method. Pharmacokinetic parameters were estimated using noncompartmental analysis (Phoenix WinNonlin; Certara LP, St Louis, MO).
Immunogenicity and neutralizing antibody assays
Patients were assessed for immunogenicity to moxetumomab pasudotox using a solid-phase enzyme-linked immunosorbent assay that measures the capacity of antidrug antibodies to inhibit the binding of moxetumomab pasudotox to CD22. Samples were obtained precycle, before the sixth dose of each cycle, and at end of treatment.
Response criteria
Statistical analyses
Fifty-five patients who were enrolled and received ≥1 dose of study drug were included in the statistical summaries and safety analyses. The DLT-evaluable population comprised patients who received any treatment with moxetumomab pasudotox and completed the DLT evaluation period or discontinued study drug because of DLT. Those who did not complete the disease assessment period in the first cycle of therapy were not evaluable for response unless they discontinued treatment because of progressive disease.
Data analyses were conducted using the SAS System (version ≥8.2; SAS Institute Inc., Cary, NC). Pharmacokinetic analyses were performed using WinNonLin software (Pharsight, Princeton, NJ). Time to response and duration of response were calculated for the subgroup of patients with CRs and PRs. Categorical data were summarized by the number and percentage of patients in each category. Continuous variables were summarized by descriptive statistics, including mean, standard deviation, median, and minimum and maximum values. Fisher’s exact test was used in statistical comparisons in proportions between 2 groups.
Results
Fifty-five patients age 1 to 25 years (median, 13 years) with ALL received moxetumomab pasudotox (Table 2). All had received prior chemotherapy, 26 (47%) had undergone allogeneic hematopoietic stem-cell transplantation, 2 had received blinatumomab, and 2 had received CD19-targeting chimeric antigen receptor (CAR) T cells. The median number of administered cycles was 1 (range, 1-4 cycles); the mean was 1.6; 40% of patients received ≥ 2 cycles of treatment.
MTCD determination
The MTCD was defined as the highest dose and number of moxetumomab pasudotox doses that could be administered safely based on safety assessments (Table 3). Six patients developed DLTs: 2 of 4 patients receiving dose-level 4A (30 µg/kg every other day for 6 doses) had dose-limiting CLS (grade 3 or 4); 1 of 8 receiving dose-level 5B (40 µg/kg every other day for 6 doses) had refractory hypercalcemia in the setting of active leukemia (patient died as a result of cardiac arrhythmia; no other cases of hypercalcemia were observed); 1 of 11 receiving dose-level 5C (32 [40] µg/kg every other day for 10 doses) had a DLT of grade 4 hepatobiliary disorder (with subsequent death resulting from multiorgan failure); and 2 of 14 receiving dose-level 6C (50 µg/kg every other day for 10 doses) had a DLT of grade 4 HUS. Additional HUS/thrombotic microangiopathy (TMA)/HUS-like events occurred during treatment during the expansion phases of dose-level 5C (32 [40] µg/kg every other day for 10 doses; 2 of 11 patients) and dose-level 6C (50 µg/kg every other day for 10 doses; 6 of 14 patients; supplemental Table 2). Although these events did not meet criteria for DLT, and they occurred beyond cycle 1, these dose levels were deemed to have exceeded the MCTD based on the additional HUS-related events. Because administration of moxetumomab pasudotox on a 10-dose schedule exceeded the MTCD at 2 dose levels that were tolerable according to the 6-dose schedule, the 10-dose schedule was abandoned. Dose-level 6B (50 µg/kg every other day for 6 doses) was determined to be the MTCD and was the recommended phase 2 dose for further evaluation in relapsed or refractory pediatric ALL. After a protocol amendment to include dexamethasone prophylaxis, no additional cases of dose-limiting CLS were observed after cohort A.
Safety
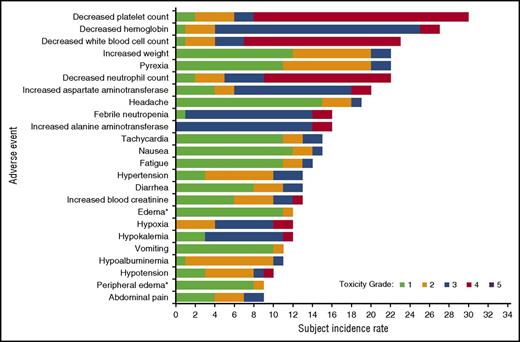
Most treatment-related AEs were grade 1 or 2 in severity (78%) and reversible (Figure 1); the most common treatment-related AEs included elevated ALT and AST, weight increase, hypoalbuminemia, and edema. Most of the patients with hypoalbuminemia and/or edema were not classified as having CLS. As noted in “MTCD determination,” 2 patients in cohort A developed CLS that was dose limiting. In total, 4 patients had an AE of CLS, 2 of whom had hypoalbuminemia, 1 of whom had edema, and 1 of whom had both hypoalbuminemia and edema.
Treatment-emergent AEs (by preferred term) reported in ≥15% of patients in the DLT-evaluable population, regardless of attribution. Each AE was counted only once per patient, regardless of the number of events observed in an individual patient. Events are rank ordered by overall frequency, and maximum event grade is displayed. *AEs were coded by preferred term by the reporting investigator according to MedDRA, which includes edema and peripheral edema.
Treatment-emergent AEs (by preferred term) reported in ≥15% of patients in the DLT-evaluable population, regardless of attribution. Each AE was counted only once per patient, regardless of the number of events observed in an individual patient. Events are rank ordered by overall frequency, and maximum event grade is displayed. *AEs were coded by preferred term by the reporting investigator according to MedDRA, which includes edema and peripheral edema.
In total, 96 serious AEs occurred in 37 patients (67%); 32 of these in 14 patients (25%) were treatment related. Diagnostic criteria for HUS were met in 5 (13%) of 55 patients, with 2 more patients experiencing an AE of TMA and 3 additional patients manifesting HUS-like features (supplemental Table 2). Although doses of 50 µg/kg every other day on a 10-dose schedule were tolerated by some patients for as many as 4 cycles, HUS was more frequent at higher dose levels and on the 10-dose schedule. Plasmapheresis was employed in both cases of grade 4 HUS; 1 case resolved gradually, whereas the other patient, with rapidly progressive ALL, had persistent renal insufficiency at time of death resulting from leukemia. In all other cases, the HUS and HUS-related events resolved with supportive measures (excluding plasmapheresis) and discontinuation of moxetumomab pasudotox.
Pharmacokinetics
A dose-dependent increase in exposure was observed across the 20- to 50-µg/kg dose range, with wide interpatient variation in pharmacokinetic parameters (Figure 2; supplemental Table 3). No systemic accumulation was observed after multiple doses. There was no obvious correlation of pharmacokinetic parameters with toxicity.
Mean concentration-time profiles for cycle 1 (dose 1). Error bars represent standard error of the mean. LLOQ, lower limit of quantification. *Manufacturing process change: 32 µg/kg bioactively equivalent to 40 µg/kg of prior product.
Mean concentration-time profiles for cycle 1 (dose 1). Error bars represent standard error of the mean. LLOQ, lower limit of quantification. *Manufacturing process change: 32 µg/kg bioactively equivalent to 40 µg/kg of prior product.
Immunogenicity
Twelve of 55 patients had a positive neutralizing antibody screen, defined as the capacity to inhibit ≥50% of moxetumomab pasudotox binding to CD22 before moxetumomab pasudotox dosing. Six of these 12 patients maintained neutralizing antibody-positive status during drug treatment. Five additional patients developed neutralizing antibodies with moxetumomab pasudotox treatment after a median of 1.0 treatment cycle (range, 1-3 cycles). Development of neutralizing antibodies did not seem to correlate with disease response or toxicity (supplemental Table 4).
Clinical activity
Forty-seven patients were evaluable for response; 8 patients received study drug but were removed from the study early and did not have posttreatment disease reassessment (because of DLT in 4 patients, underlying complications of ALL in 3 patients, and use of corticosteroids as part of management of HUS-like symptoms in 1 patient). Eleven patients (23%) achieved a CRc, 5 of whom were MRD negative by flow cytometry (Table 4; Figure 3; supplemental Table 4). Three of the 5 patients who achieved MRD-negative CRc proceeded directly to second stem-cell transplantation. Of the other 2 patients, 1 became MRD positive and proceeded to CAR T-cell therapy followed by second stem-cell transplantation. The other, who had relapsed after receiving 2 prior transplants, developed neutralizing antibodies and remained MRD negative during chemotherapy. Median time to CRc was 0.72 months (range, 0.49-1.61 months), and median CRc duration was 1.64 months (range, 0.03-1.97 months). Four of 47 evaluable patients achieved PR, yielding an overall objective response rate of 32% (Table 4). Among patients who relapsed after prior stem-cell transplantation (n = 22), 9 (41%) achieved CRc, 5 of whom were MRD negative, and 1 achieved PR, for an overall objective response rate of 45%. No association was observed between baseline CD22 expression on leukemic blasts and clinical response (supplemental Table 4). Median progression-free survival among the 55 treated patients was 1.6 months (range, 0.2-17.3 months).
Responses: patient examples. (A) Flow cytometric CRc in patients #14 and #37. Pretreatment (left panels) bone marrow aspirates demonstrated ALL blasts expressing abnormally bright CD20 and CD10. Posttreatment (right panels) bone marrow aspirates demonstrated only pre–B cells with a normal maturation profile based on CD20 and CD10 expression. Both patients achieved CRc (#14, CR; #37, CR incomplete blood count recovery [CRi]) after cycle 1 and were MRD negative after cycle 2. The limit of detection for this assay was 0.004%. (B) Patient #2: pretreatment (left panel) and posttreatment (right panel) bone marrow biopsies revealed decreased blast infiltration with 1 cycle of therapy (original magnification ×100; hematoxylin and eosin stain). Patient achieved PR after cycle 1 and CRi after cycle 2. (C) Patient #22: pretreatment (left panel) and posttreatment (right panel) fluorodeoxyglucose positron emission tomography scans show marked reduction in areas of uptake after 2 cycles of therapy.
Responses: patient examples. (A) Flow cytometric CRc in patients #14 and #37. Pretreatment (left panels) bone marrow aspirates demonstrated ALL blasts expressing abnormally bright CD20 and CD10. Posttreatment (right panels) bone marrow aspirates demonstrated only pre–B cells with a normal maturation profile based on CD20 and CD10 expression. Both patients achieved CRc (#14, CR; #37, CR incomplete blood count recovery [CRi]) after cycle 1 and were MRD negative after cycle 2. The limit of detection for this assay was 0.004%. (B) Patient #2: pretreatment (left panel) and posttreatment (right panel) bone marrow biopsies revealed decreased blast infiltration with 1 cycle of therapy (original magnification ×100; hematoxylin and eosin stain). Patient achieved PR after cycle 1 and CRi after cycle 2. (C) Patient #22: pretreatment (left panel) and posttreatment (right panel) fluorodeoxyglucose positron emission tomography scans show marked reduction in areas of uptake after 2 cycles of therapy.
Discussion
Moxetumomab pasudotox, a second-generation recombinant immunotoxin, targets CD22.11,12 It is highly active, with an acceptable safety profile in adults with hairy cell leukemia, wherein a phase 3 dose of 40 µg/kg (process 3) every other day for 3 doses every 28 days has been defined.18 Our pediatric phase 1 trial demonstrated activity in chemotherapy-resistant ALL, with a 32% objective response rate in 47 evaluable patients. Notably, 5 of 11 of those who achieved CRc became MRD negative; 4 of these patients subsequently underwent second allogeneic stem-cell transplantation, indicating the potential utility of moxetumomab pasudotox as a bridge to transplantation. Also notable was the high response rate in patients who had relapsed after prior stem-cell transplantation, with a CRc rate of 41% (5 of 9 MRD negative) and an overall objective response rate of 45%. The outlook for relapsed ALL after transplantation is guarded, and new therapies are needed to improve outcomes.19,20 These results support studies of moxetumomab pasudotox in the setting of posttransplantation relapse.
The basis for the observed variability in patient responses in this trial is largely unexplained. CD22 expression is a determinant of response to BL22 in vitro21 ; however, there was no apparent influence of antigen density in our trial, with the exception of 2 patients with KMT2A (MLL)-rearranged infant ALL. Blasts from individuals with 11q23 (KMT2A) rearrangement may have low CD22 site density and/or subpopulations of CD22− blasts.6 Two such patients in this trial experienced transient reduction in peripheral blast percentage that was followed by disease progression resulting from expansion of CD22−/dim populations. CD22 expression was otherwise maintained in patients with residual or recurrent disease (data not shown), in contrast to the loss of surface antigen expression seen after CD19-targeted therapies with CAR-transduced T cells and bispecific T-cell–engaging antibodies.22,23 We previously showed that disease burden in children with ALL can influence the pharmacology of anti-CD22 immunotoxins, with binding by CD22+ blasts resulting in rapid drug clearance.10 It is noteworthy that wide interpatient pharmacokinetic variability was observed in this trial. Drug neutralization is another known cause of resistance to immunotoxin therapy.10 Notably, intermittent exposure to sublethal doses of moxetumomab pasudotox in vitro can lead to reversible epigenetic changes that confer resistance in ALL cell lines.24,25 This potential mechanism of resistance has not yet been explored in patients. Finally, we recently demonstrated wide interpatient variability in the exposure time required to induce cytotoxicity of ALL blasts by moxetumomab pasudotox.26 Thus, the activity of moxetumomab pasudotox in patients with ALL might be improved by changing administration from bolus to continuous infusion, especially given the short elimination half-life observed in this study (supplemental Table 3). Importantly, dexamethasone cotreatment is unlikely to have contributed to the antileukemia activity observed in this trial. Dexamethasone was employed for CLS prophylaxis around the first 6 doses of moxetumomab pasudotox in cycle 1 in cohorts B and C only. Thus, the CRc observed in cohort A occurred without steroid treatment. Furthermore, some patients had continued objective responses in subsequent cycles, without concurrent dexamethasone. Finally, all patients had multiply relapsed or refractory disease and had received corticosteroids as part of prior therapy.
Although moxetumomab pasudotox was generally well tolerated, AEs, which were reversible in almost all cases, were common. Most notably, HUS/TMA developed in 7 patients; another 3 had HUS-like features. The mechanism of this toxicity remains unknown. HUS has been observed in adults after treatment with BL22 and moxetumomab pasudotox.18,27,28 The risk of HUS in our study may have been linked to dose and dose intensity. Additionally, HUS seemed to occur with higher frequency in patients who had previously undergone stem-cell transplantation (8 of 26 vs 2 of 29; P = .0346 by Fisher’s exact test), and TMA is a well-described posttransplantation complication.29 Importantly, HUS/TMA resolved after study drug discontinuation in all but 1 patient. HUS/TMA did not seem to prohibit subsequent treatment with stem-cell transplantation or T-cell therapy, because 2 patients with grade 2 HUS/TMA received second allogeneic stem-cell transplants as next therapy after achieving CRc in this trial, and 1 with grade 4 HUS subsequently received CAR-transduced T-cell therapy.
The other important treatment-related toxicity observed in this trial, CLS, was dose limiting in 2 cohort A patients; both cases were fully reversible. The risk and severity of CLS seemed to be reduced with dexamethasone prophylaxis during the first 6 doses of cycle 1. Only 1 subsequent patient treated at dose-level 4B developed grade 2 treatment-related CLS, which was fully reversible despite continuation of moxetumomab pasudotox. All CLS cases developed during the first cycle of therapy. Notably, neither CLS nor CR were observed in the predecessor BL22 trial.10 This raises the possibility that soluble mediators associated with blast-cell lysis may have contributed to the development of CLS, as seen with other agents used in the treatment of childhood ALL.30
Moxetumomab pasudotox is 1 of a growing number of monoclonal antibody–based therapies that target CD22. This recombinant immunotoxin is composed of an anti-CD22 antibody variable fragment that has been disulfide stabilized and fused to a fragment of Pseudomonas exotoxin A. In comparison with whole antibodies or larger antibody fragments (eg, Fab), variable fragment constructs have improved tissue penetration and reduced immunogenicity.31,32 Single-agent activity of the unconjugated anti-CD22 antibody epratuzumab against ALL seems to be limited, with no objective responses (0 of 15) observed in children with relapsed ALL.33 The anti-CD22 drug conjugate inotuzumab ozogamicin has shown activity in adults and children with relapsed ALL, with reported CRc rates of approximately 80% in adults and 60% (3 of 5 responders, with 1 CR and 2 CRs with incomplete blood count recovery) in children, although with a relatively high incidence of hepatic venoocclusive disease.34,35 CAR-transduced T cells targeting CD22 have been developed and are active in preclinical models of ALL.36 Importantly, CAR-transduced T-cell therapy is associated with a high risk of severe cytokine release syndrome and requires complex patient-specific manufacturing.23 In contrast, moxetumomab pasudotox represents an off-the-shelf agent.
In conclusion, this trial provides proof of principle that moxetumomab pasudotox is active against CD22-expressing ALL and can overcome chemotherapy resistance, inducing MRD-negative remissions in children with multiply relapsed or refractory disease, with a manageable safety profile. Further clinical evaluation of the recommended phase 2 dose and schedule is warranted. Such a study was recently conducted and will be reported separately.
Presented in part in abstract form at the 105th annual meeting of the American Association for Cancer Research, San Diego, CA, 5-9 April 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the clinical trial participants and their families, the referring physicians and care teams, and the trial site clinical research teams, faculty, and staff. MedImmune team members contributing to the trial include Pandy Opima, Carolyn Pendry, Alan Meier, Fatemeh Tavakkoli, Jody Koepp-Norris, Linda Chang, Nai Shun Yao, and Guozhi Gao. The authors thank Amy Zannikos of Peloton Advantage, Parsippany, NJ, for medical writing and editorial support, which was funded by MedImmune.
This research was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health (NIH), a cooperative research and development agreement between the NIH, NCI, and MedImmune, and by NCI award P30CA014089. This clinical study was sponsored by MedImmune.
Authorship
Contribution: A.S.W. and I.P. designed the study; A.S.W., N.N.S., D.B., L.B.S., J.A.W., and W.S. provided patients or study materials; A.S.W., N.N.S., M.C.L., M.L., J.Y., W.S., I.P., R.J.K., D.B., and M.S.-S. participated in the collection and assembly of data and in data analysis and interpretation; A.S.W. wrote the first draft of the manuscript; and all authors participated in the critical review and revision of this manuscript and provided approval of the manuscript for submission.
Conflict-of-interest disclosure: A.S.W. has received honoraria and travel support from MedImmune. A.S.W., R.J.K., I.P., and N.N.S. have participated in research through a cooperative research and development agreement between MedImmune and the National Institutes of Health (NIH). A.S.W., R.J.K., and I.P. are co-inventors and hold patents assigned to the NIH for the investigational product. D.B. has served as a consultant and received travel support from Amgen (funds received by her affiliation). J.A.W. has received research funding from MedImmune. M.L., J.Y., and M.C.L. are employees of MedImmune. The remaining authors declare no competing financial interests.
Correspondence: Alan S. Wayne, Children’s Center for Cancer and Blood Diseases, Children’s Hospital Los Angeles, University of Southern California, 4650 Sunset Blvd, Mailstop #54, Los Angeles, CA 90027; e-mail: awayne@chla.usc.edu.


![Figure 3. Responses: patient examples. (A) Flow cytometric CRc in patients #14 and #37. Pretreatment (left panels) bone marrow aspirates demonstrated ALL blasts expressing abnormally bright CD20 and CD10. Posttreatment (right panels) bone marrow aspirates demonstrated only pre–B cells with a normal maturation profile based on CD20 and CD10 expression. Both patients achieved CRc (#14, CR; #37, CR incomplete blood count recovery [CRi]) after cycle 1 and were MRD negative after cycle 2. The limit of detection for this assay was 0.004%. (B) Patient #2: pretreatment (left panel) and posttreatment (right panel) bone marrow biopsies revealed decreased blast infiltration with 1 cycle of therapy (original magnification ×100; hematoxylin and eosin stain). Patient achieved PR after cycle 1 and CRi after cycle 2. (C) Patient #22: pretreatment (left panel) and posttreatment (right panel) fluorodeoxyglucose positron emission tomography scans show marked reduction in areas of uptake after 2 cycles of therapy.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/14/10.1182_blood-2017-02-749101/4/m_blood749101f3.jpeg?Expires=1765125404&Signature=kiy6WRhWl~1U0o0TEjEs4XROVxBwBWB2R26kRUKHNRXIvoup86MVjPtx9fiMMIRsa0acazpP8x1cThvlH001ME~aAMxCxk5MOGzHHt9w2YkS0K8UN61~fvUb2MKIMqWT8QZxsTBwamJJsgnEilZj4DkwSPyu50j~b8qqftd7NEAmiAmUP357yh3tT64dQ8DsNC4XoMZyRryZepF4QAli4uyfFNBdWuBvJHxTbioOEfbF~xqVUQvkqoQILc-u4fqg84UDYmdtHmH3F6TfWySZxOb-7H8qJT9jsIJ3qxWigC5frL3Xtqd~gITYvKsWwid81moPgVmADzAOMDAqontZ5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
