In this issue of Blood, Rosendaal and colleagues provide important new data on the interplay between genetics and treatment that impacts inhibitor formation in hemophilia A.1
The major complication of hemophilia treatment is the formation of neutralizing antibodies (inhibitors) to infused factor replacement therapy. This occurs in ∼30% of boys with severe hemophilia A, usually within 9 to 12 exposure days, although some risk continues throughout their lifetimes.2,3 To date, approved therapies for patients with inhibitors are suboptimal, and these patients have poorer outcomes than those treated without inhibitors.4 Thus, a major effort in hemophilia research has been to define risk factors for inhibitor formation and determine optimal therapy to lessen inhibitor risk.
Risk factors for inhibitor formation in hemophilia A include the underlying pathogenic DNA variant (mutation), family history, ethnicity, and circumstances of and around first exposure.5 Increased risk, if any, attributable to the type of replacement product has been unclear. Factor VIII is normally stabilized in circulation by binding to von Willebrand factor (VWF). Many case control and cohort studies, but not all, have found a lower risk of inhibitor formation in patients receiving plasma-derived factor VIII (pdFVIII), which also contains VWF, compared with the risk associated with recombinant factor VIII (rFVIII).6-8
The Survey of Inhibitors in Plasma-Product Exposed Toddlers (SIPPET) was a prospective trial comparing inhibitor development in children without prior FVIII concentrate treatment who were randomly assigned in a 1:1 ratio to receive either VWF-containing pdFVIII or rFVIII replacement product.9 In boys receiving pdFVIII, 26.8% developed inhibitors compared with 44.5% in the rFVIII arm. In multivariate analysis the hazard ratio for developing an inhibitor was 1.95 (95% confidence interval [CI], 1.21-3.15) for low-titer (0.4-4.9 Bethesda units [BU]) and 1.73 (95% CI, 0.97-3.10) for high-titer (≥5 BU) inhibitors in those receiving rFVIII compared with pdFVIII. While there have been some criticisms of the study, the data compel us to examine treatment approaches to replacement therapy for young children with severe hemophilia A.
Most children in the United States and many other countries are routinely treated with rFVIII replacement therapy. This provides a theoretical safety margin over virally inactivated pdFVIII products and also provides comparative ease in handling and administration. Identifying groups where pdFVIII would provide the most benefit would help personalize care and continue current treatment approaches in many children.
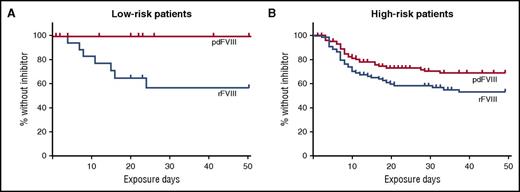
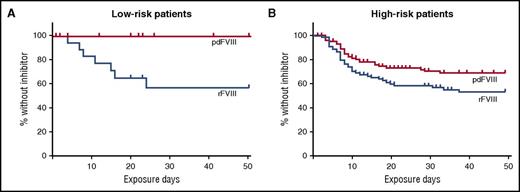
In the article by Rosendaal and colleagues, the investigators further analyzed the SIPPET data to evaluate the role of the patient’s underlying F8 DNA variant in inhibitor development by factor product used. F8 variants that place patients at a relative higher risk of inhibitor development include nonsense or frameshift variants, large deletions, and intron 22 or intron 1 inversions. The results of their analysis are shown in the figure. While there was not a significant difference in inhibitor development in those with high-risk F8 variants, there was a marked difference in individuals with low-risk variants. As expected, there were fewer inhibitors in the low-risk F8 variant group (24% vs 38%); 7 of the 38 patients in this group developed an inhibitor, 4 of which were high titer. In the low-risk F8 variant group, only patients who received rFVIII developed inhibitors. While the numbers are small, the results are still striking.
Kaplan-Meier curves show the cumulative incidence of being without an inhibitor by treatment type in patients with F8 DNA variants that put them at low risk (A) or high risk (B) of developing a factor VIII inhibitor. This figure has been adapted from Figure 1 in the article by Rosendaal et al that begins on page 1757.
Kaplan-Meier curves show the cumulative incidence of being without an inhibitor by treatment type in patients with F8 DNA variants that put them at low risk (A) or high risk (B) of developing a factor VIII inhibitor. This figure has been adapted from Figure 1 in the article by Rosendaal et al that begins on page 1757.
How do we use this information to guide therapy? An underlying F8 variant that results in no protein production appears to be a major driver of inhibitor formation. However, in patients with less disruptive variants, other genetic and environmental factors have greater influence. It is in those patients that attention to modulating risk factors may have greater impact. This hypothesis is consistent with findings of the RODIN study that intensity of treatment and early prophylaxis had the most impact on inhibitor development in patients with low-risk F8 variants.10
In order to work effectively with parents in choosing the best factor product for their child, providers need a full understanding of the risks of inhibitor development. The data from the SIPPET analysis and other studies will help to inform that important decision-making process.
Conflict-of-interest disclosure: The author declares no competing financial interests.