To the editor:
Extranodal marginal zone B-cell lymphomas of mucosa-associated lymphoid tissue (MALT) present in most cases as localized disease and can be successfully treated with local therapies or antibiotics.1-4 Moreover, patients with multicentric or extensive disease or relapsed disease may require systemic treatment with chemotherapy and/or rituximab.5-8 Also, considering the relative frequency of late relapses9 and that extragastric MALT lymphomas have an increased relapse risk,10 systemic treatment has also been used in the first-line setting. Several chemotherapy agents, anti-CD20 monoclonal antibodies, immunomodulatory agents, and their combinations have been demonstrated to be effective specifically in MALT lymphomas.11-14 Herewith, we report the long-term results of the MALT 2008-01 phase 2 trial15 using a response-adapted protocol with the combination of rituximab and bendamustine (RB) as first-line treatment of MALT lymphomas after a median follow-up of 7 years. This trial was registered at www.clinicaltrials.gov as #NCT01015248 and in EudraCT as #2008-007725-39.
MALT 2008-01 is a multicenter, prospective, nonrandomized phase 2 trial conducted in Spain by the Grupo Español de Linfomas/Trasplante de Médula Ósea (GELTAMO) network. The details of the protocol are described elsewhere15 ; briefly, patients between 18 and 85 years of age with biopsy-proven CD20+ MALT lymphoma at any extranodal site and in any stage were eligible. Newly diagnosed patients with no previous treatment to any site, patients with gastric MALT lymphoma with unequivocal active disease after failure of H pylori eradication or in subsequent relapse, and patients with cutaneous MALT lymphoma in relapse or progression after local therapy were eligible. All patients had unequivocal active disease and were in need of treatment. Initial staging and response evaluation followed the Ann Arbor system and included computed tomography, bone marrow biopsy in all cases, and specific imaging or endoscopic studies according to the primary location. The response-adapted treatment consisted of rituximab 375 mg/m2 on day 1 and bendamustine 90 mg/m2 on days 1 and 2, in cycles administered every 28 days. Response was assessed after 3 cycles of therapy and at the end of the program and was based on local investigator assessment according to the National Cancer Institute standardized response criteria for malignant lymphomas.16 Patients in complete response (CR) after 3 cycles received another cycle, for a total of 4 cycles, and those with partial response received 3 additional cycles, for a total of 6 cycles. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events (version 3.0).17 The primary end point was event-free survival (EFS), and secondary end points were overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and acute and long-term toxicities.15
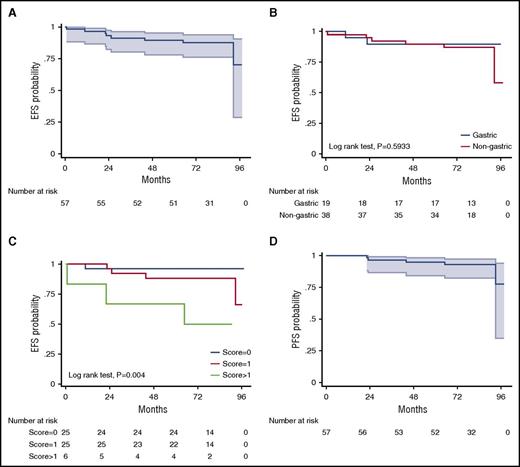
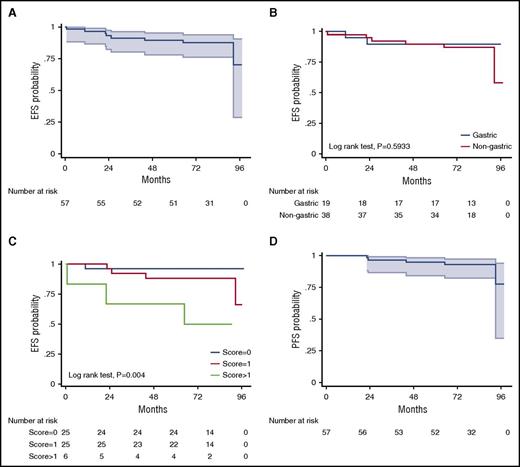
A total of 60 patients were enrolled and started treatment between May 2009 and May 2011, but 3 patients were nonevaluable for response and survival because of protocol deviations.15 The median age of the 57 evaluable patients was 62 years (range, 26-84 years); 57% of patients were women; 66% had Ann Arbor stage 1 to 2 and 34% had stage 3 to 4 disease; the site of disease was the stomach in 33%, extragastric in 57%, and multifocal in10% of patients; presence of t(11;18)(q21;q21) was identified in 9 patients (16%). After 3 cycles of RB, the ORR was 100% (95% confidence interval [CI], 93-100), with a CR/unconfirmed CR (uCR) rate of 75%. Patients with gastric involvement had a significantly higher CR/uCR rate than those with nongastric disease (90% vs 62%; P = .023), but the CR/uCR rate was not different according to Ann Arbor stage 1 to 2 vs 3 to 4 (100% vs 96%; P = .386). Only 14 patients (25%) who had not achieved CR/uCR after 3 cycles went on to receive a total of 6 cycles. At the end of therapy, the ORR and CR/uCR rate were 100% and 98%, respectively, with no significant differences according to primary site of disease. Although direct comparisons are not methodologically appropriate, 89 patients treated with R-chlorambucil in the IELSG-19 trial achieved CR rates of 91% in gastric and 72% in nongastric disease,18 rates lower than that obtained with RB (100% in both gastric and nongastric disease). Moreover, 75% of our patients achieved CR after only 3 cycles and therefore required a total of only 4 cycles. Consequently, duration of RB treatment was 4 months in most patients, a timeframe substantially shorter than the 22 weeks of treatment with R-chlorambucil, making our approach more convenient to patients. Also, the effectiveness of RB was independent of the presence or absence of t(11;18)(q21;q21),19,20 a prognostic factor that was not analyzed in the IELSG19 study.18 In the long term, after a median observation of 82 months (interquartile range, 75-90), EFS (the primary end point in both studies) was 87.7% at 7 years (95% CI, 76.0-94.0; Figure 1A), better than the 68% EFS at 5 years (95% CI, 60-76) obtained with R-chlorambucil; there was no overlapping of CIs between the studies. Interestingly, both patients with gastric and those with nongastric MALT lymphoma had excellent EFS at 7 years with RB (89.5%; [95% CI, 64.1-97.3] and 84.4% [95% CI, 66.5-93.2], respectively; P = .637; (Figure 1B); these results were superior to those reported with R-chlorambucil (5-year EFS, 77%; [95% CI, 63-86] in gastric and 63% [95% CI, 51-72] in extragastric disease). The estimated 7-year PFS with RB was 92.8% (95% CI, 81.9-97.2), with no significant differences according to primary site of disease (Figure 1D), stage, number of cycles received, or t(11;18); this result favorably compares to that obtained by in IELSG-19 (5-year PFS, 72% [95% CI, 63-79]). Relapses occurred in 5 patients: 1 patient with gastric primary site had a local relapse with diffuse large B-cell lymphoma (DLBCL) transformation, and 4 with nongastric disease also relapsed (3 at a different site from the original site of disease and 2 with DLBCL transformation).
Survival curves. EFS in all patients (A) and according to primary site of involvement (B) and MALT lymphoma international prognostic index (MALT-IPI) (C); PFS in all patients (D). Shading indicates 95% CIs (A,D).
Survival curves. EFS in all patients (A) and according to primary site of involvement (B) and MALT lymphoma international prognostic index (MALT-IPI) (C); PFS in all patients (D). Shading indicates 95% CIs (A,D).
A total of 264 cycles of treatment were administered. Overall, RB was well tolerated, with 36 patients (60%) experiencing ≥1 grade 3 or 4 AE during treatment, most commonly hematologic toxicities. Detailed AEs have been reported elsewhere.15 Beyond the first 2 years of follow-up after the end of therapy, 3 patients had opportunistic infections (1 herpes zoster, 1 cytomegalovirus, and 1 lung infection by Nocardia). No case of myelodysplastic syndrome or acute leukemia has been reported so far, but other neoplasias were observed in 3 patients (1 tongue epidermoid carcinoma, 1 gastrointestinal stromal tumor, and 1 granular lymphoproliferative disorder of natural killer cells). In addition, 3 patients had nonmelanoma skin cancers. No deaths occurred during the treatment period, but 3 patients died during follow-up, 2 as a result of causes unrelated to lymphoma or treatment and 1 as a result of DLBCL transformation.
Recently, a specific prognostic score for MALT lymphomas (called MALT-IPI) was developed from the data of the IELSG-19 study21 ; the resulting prognostic factors (age >70 years, elevated lactate dehydrogenase and disease stage >2, and presence of 0, 1, or >1) were able to discriminate 3 risk groups with significantly different EFS rates (5-year EFS, 69.8%, 55.7%, and 28.7%, respectively) and significant differences in PFS and OS. In our prospective trial, MALT-IPI also identified 3 risk groups with significantly different 7-year EFS (96%, 88%, and 50%, respectively) and PFS rates (100%, 91.8%, and 66.7%); (Table 1; Figure 1C).
In summary, this first-line response-adapted strategy with the combination RB for MALT lymphoma at any site or stage has shown extremely good efficacy with excellent short- and long-term outcomes. In addition, toxicity in both the short and long term is also favorable (probably related to the short duration of this treatment in most patients), making this scheme a standard treatment for patients with MALT lymphoma requiring systemic therapy.
Authorship
Acknowledgments: The authors thank the patients and investigators who participated in this study.
This study was supported (in part) by Grupo Español de Linfomas/Trasplante de Médula Ósea (GELTAMO) and research funding from Mundipharma Spain; Roche Pharma Spain provided rituximab.
Contribution: A.S. and C.M. were responsible for conception and design; A.S., E.D.-D., C.P., C.N., J.B., A.M., M.C., J.L.B., J.M.S., J.F.T., M.J.R., J.P., C.G., J.J.S.-B., L.P., R.A., E.C., D.C., and C.M. for provision of study materials and/or patients; J.F.G. and M.G. for pathological review; and A.S. and C.M. for collection and assembly of data, data analysis and interpretation, and manuscript writing; all authors provided final approval of manuscript.
Conflict-of-interest disclosure: A.S. has received an honorarium for advisory board participation from Roche and Mundipharma; D.C. has received an honorarium for advisory board participation from Roche, Mundipharma, and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Antonio Salar, Department of Hematology, Hospital del Mar, Passeig Maritim 25-29, 08003 Barcelona, Spain; e-mail: asalar@parcdesalutmar.cat.