Key Points
The addition of rituximab to corticosteroid and CsA is safe and effective for first-line treatment of cGVHD.
Resistance of PD-L1hi B cells to anti-CD20 depletion may lead to the suppression of activated Tfh cells and cGVHD control.
Abstract
Chronic graft-versus-host disease (cGVHD) is the main cause of late nonrelapse mortality and morbidity after allogeneic stem cell transplantation (allo-SCT). To improve such patients’ outcomes, we conducted a phase 2, prospective, multicenter trial to test the efficacy of the addition of rituximab to corticosteroids (CSs) and cyclosporine A (CsA) as first-line therapy for newly diagnosed cGVHD after allo-SCT. Twenty-four patients (median age, 47 years) with mild (n = 2), moderate (n = 7), or severe (n = 15) cGVHD were included. All patients received rituximab 375 mg/m2 weekly for 4 weeks, followed by a second course 1 month later for patients with partial response. Twenty of 24 patients (83%) were in response at 1 year. Furthermore, among 19 evaluable patients, 14 (74%) were off CSs. The estimated 1-year overall survival was 83%, and the 1-year cumulative incidence of nonrelapse mortality was 14%. One patient died of progressive multifocal leukoencephalopathy. Although PD-L1hi naive B cells were significantly decreased at diagnosis of cGVHD, they increased after anti-CD20 B-cell depletion. In contrast, activated ICOShi PD-1hi circulating T follicular helper (Tfh) cells decreased after rituximab treatment. Overall, the addition of rituximab to corticosteroid and CsA appeared to be safe and effective for first-line treatment of cGVHD. Furthermore, our data suggest that this efficacy may be in part related to an effect on PD-L1hi B cells and Tfh cells. This study was registered at www.clinicaltrials.gov as identifier NCT01135641.
Introduction
Despite global improvement in the outcome of patients undergoing allogeneic stem cell transplantation (allo-SCT),1,2 chronic graft-versus-host disease (cGVHD) continues to account for significant morbidity and mortality after the procedure.2,3 Standard first-line systemic treatment of cGVHD comprises orally administered corticosteroids (CSs [prednisolone, 1 mg/kg]) and cyclosporine A (CsA).4 Attempts to identify novel agents or strategies to improve initial therapy for cGVHD have not improved on the combination of CSs and CsA.4-6 Furthermore, long-term immunosuppressive treatment, up to 2 years, is generally required to control the disease.7,8 The longer duration of cGVHD made it the main cause of late morbidity, mortality, and impaired quality of life (QOL). Furthermore, long-term glucocorticoid therapy is associated with a wide range of side effects, impairs immune function, and increases the risk of opportunistic infection.9 Therefore, the evaluation of new immunosuppressive treatment with low infectious morbidity and mortality for cGVHD first-line treatment is urgently needed.
Failure to improve cGVHD therapy can be partly attributed to an incomplete understanding of its pathophysiology. Although cGVHD is traditionally thought to be mediated by donor-derived alloreactive T cells, there is mounting evidence implicating B cells.10 After allo-SCT, recovery of peripheral B cell occurs in the setting of ubiquitous foreign antigens and high levels of plasma B-cell activating factor.10-12 This promotes the survival of activated, potentially allo- and autoreactive B cells.10 Therefore, cGVHD is associated with altered B-cell homeostasis and increased B-cell activating factor to B-cell ratios.10,12 Of note, in sex-mismatched allo-SCT, the development of anti-HY antibodies is strongly correlated with cGVHD.13
Overall, these data suggest that B-cell directed therapy may be effective for treatment of cGVHD. The efficacy of anti–B-cell therapy using rituximab, an anti-CD20 monoclonal antibody, suggested in 2003,14 has been reported for second-line treatment of steroid-refractory cGVHD.15-19 More recently, several groups showed that prophylactic rituximab in the posttransplantation period decreased the rate of cGVHD.20,21 As for first-line treatment, Solomon et al reported that although a CS-free treatment of cGVHD incorporating rituximab induced a significant reduction of cGVHD, it was associated with a recurrence rate of 37%.22
We conducted a multicenter, prospective phase 2 study evaluating the addition of rituximab to CSs and CsA as first-line therapy for newly diagnosed cGVHD after allo-SCT. We hypothesized that this combination would increase the overall response rate and enable a more rapid and effective CS taper.
Patients and methods
Study design and inclusion criteria
This prospective, multicenter, phase 2 study included 24 patients who presented with cGVHD after allo-SCT between 2008 and 2012. The study was approved by each institutional review board of the participating centers, the Comité de Protection des Personnes de Tours (CPP Ouest-1, reference 2011-R11), and the national Agence Française de Sécurité Sanitaire des Produits de Santé. This trial was registered at ClinicalTrials.gov as identifier NCT01135641. Informed consent from patients was obtained before inclusion in the study.
Patients >18 years of age who had received a first allo-SCT for a hematological malignancy were eligible if they had a confirmed diagnosis of a first episode of cGVHD requiring systemic immunosuppressive therapy. cGVHD was defined according to National Institutes of Health (NIH) criteria. Any hematopoietic stem cell source and type of donor were authorized. Patients who had received reduced-intensity or myeloablative conditioning were eligible.
Exclusion criteria included patients developing acute graft-versus-host-disease (GVHD; (whether early or late-onset form, including overlap syndrome), cGVHD not requiring systemic immunosuppressive therapy, treatment with prednisone (or equivalent) at doses ≥1 mg/kg per day, occurrence of cGVHD following donor lymphocyte infusion, uncontrolled systemic infection associated with an increased risk of death within 1 month, severe neurological or psychiatric disorder, and pregnancy.
Treatment
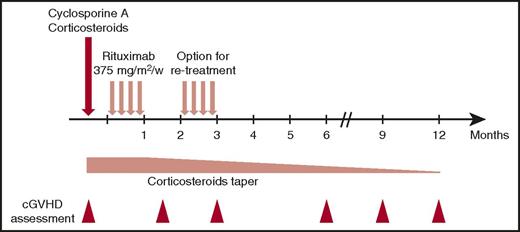
As schematically shown in Figure 1, once the diagnosis of cGVHD requiring systemic therapy was established, patients received IV rituximab 375 mg/m2 weekly for 4 consecutive weeks in addition to CsA and CSs 1 mg/kg per day equivalent prednisone. Rituximab was started within 14 days of starting CSs. Patients who had a complete response (CR) after the first cycle of rituximab did not receiving a second cycle, whereas those in partial response (PR) were eligible to receive a second cycle of rituximab at 375 mg/m2 weekly for 4 weeks, after a delay of 8 weeks from the first infusion of rituximab. Additionally, patients who achieved CR after an initial treatment with 1 cycle of rituximab and who subsequently relapsed were eligible to receive a second cycle.
CsA was resumed at 6 mg/kg administered orally twice daily or continued for patients already treated with CsA at the time of enrollment. CsA plasma levels were monitored at the managing physician’s discretion to allow for dose adjustment and maintenance within the therapeutic range (200-400 ng/mL). The initial CS dose was 1 mg/kg per day of a prednisone equivalent, and this was maintained for at least 2 weeks, after which a tapering schedule was commenced up until response assessment at 6 weeks. Thereafter, CS tapering was pursued at the managing physician’s discretion according to response. The protocol provided guidelines for tapering CSs, with a recommendation for it to be done before tapering other immunosuppressive medications. Toxicity associated with the administration of CSs was managed according to standard practice in each transplant center. Prophylaxis against infection included acyclovir or valacyclovir for herpes simplex virus and trimethoprim/sulfamethoxazole (substituted by atovaquone or inhaled pentamidine in case of allergy) for Pneumocystis jirovecii. Cytomegalovirus (CMV)-seropositive patients and patients with a CMV-seropositive donor were monitored to initiate preemptive valganciclovir therapy. No specific antifungal prophylaxis was recommended; this was administered at the discretion of the managing physician according to the transplant center’s policies.
Clinical outcome and GVHD assessment
The primary end point of the study was the overall response rate (ie, CR and PR) of cGVHD at 12 months after its diagnosis, and first-line treatment with the combination of CsA, prednisone, and rituximab. The cGVHD clinical stage was assessed at 6 weeks and at 3, 6, 9, and 12 months postinclusion, according to the NIH grading system.23 CR was defined as complete disappearance of any sign of cGVHD.24 PR was defined as improvement in ≥1 organ or site without progression in any other organ or site.24 Cases where death or dropout occurred with lack of follow-up information after response of cGVHD to treatment but before 12 months were not used to negate categorization for efficacy success if the treatment had been effective in controlling cGVHD. The secondary end points were the percentage of reduction of CS dosage at 12 months, the percentage of patients with treatment failure, the cumulative incidence of nonrelapse mortality (NRM) at 12 months, and the descriptive analysis of QOL parameters according to the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30)25 and the Lee Chronic GVHD Symptom Scale.26 Treatment failure was defined as lack of a CR or PR at week 6 or requirement of alternative therapy before week 6 or death before week 6.
Sample collection and processing
For 16 patients, peripheral blood samples were collected in EDTA tubes at inclusion and at 12 months. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation. Samples were centrifuged for 30 minutes at 1500 rpm, and plasma and PBMCs were collected and then cryopreserved. In addition, 5 mL of blood was collected in plain tubes at each time point and centrifuged for 10 min at 400g, and serum was collected and cryopreserved. Peripheral blood samples were collected from healthy donors (Etablissement Français du Sang Pays de Loire) after obtaining informed consent. Furthermore, a control cohort of patients who received allo-SCT without developing cGVHD was assessed. Comparisons of the patients and transplant characteristics are listed in supplemental Table 1, available on the Blood Web site. In the control cohort, samples were harvested at a median of 359 days (range, 179-378 days) after transplant compared with 240 days (range, 132-1017 days) in the group of patients at diagnosis of cGVHD (study population group, P = .37).
Flow cytometry
The phenotypes of T and B cells were determined on frozen aliquots of PBMCs. These were first stained with eFluor506 Fixable Viability Dye (eBioscience, Paris, France), according to the manufacturer’s instructions, and subsequently marked with fluorochrome-conjugated antibodies to characterize the T and B cells. The following antibodies were used: fluorescein isothiocyanate–conjugated ICOS, PE-conjugated PD-1, PerCP-Cy5.5–conjugated CD8, allophycocyanin (APC)-R700–conjugated CD4, APC-Cy7–conjugated CD3, BUV395-conjugated CD45RO, BV421-conjugated CXCR5, fluorescein isothiocyanate–conjugated CD19, PeCy7-conjugated immunoglobulin D (IgD), APC-conjugated CD27, BUV395-conjugated CD24, BV650-conjugated PDL1, and BV786-conjugated CD38, all from BD Biosciences. Analyses were performed by using a LSR Fortessa (BD Biosciences, Le Pont de Claix, France) and FlowJo version 8.8.6 software (Tree Star).
Cytokine measurement by enzyme-linked immunosorbent assay
CXCL13 concentrations were measured in plasma by using specific enzyme-linked immunosorbent assay sets, purchased from R&D Systems (Minneapolis, MN), according to the manufacturer’s instructions.
Statistical analysis
The statistical plan is provided in supplemental Methods. Continuous variables are presented as medians (ranges), and categorical variables as counts and percentages. A probability of overall survival (OS) was calculated by using the Kaplan-Meier estimates. NRM was calculated using the cumulative incidence procedure, and relapse or progression was considered as the competing event. The Mann-Whitney nonparametric test was used to compare B and T follicular helper (Tfh) cell populations between groups. Statistical analyses were performed with SPSS software version 19 (SPSS, Inc/IBM, Armonk, NY) and R version 2.13.2 (R Development Core Team, Vienna, Austria) software packages.
Results
Patient, donor, and transplant characteristics
The patient and donor characteristics are summarized in Table 1. The median age of the recipient was 47 years (range, 23-63 years). A total of 7 patients (29%) were male with a female donor. Six patients (25%) underwent transplant for myeloid malignancies, and 18 (75%) for lymphoid malignancies. Ten donors (42%) were HLA-identical siblings, 9 (37%) were matched unrelated donors, and 5 (21%) were mismatched unrelated donors. The stem cell source was bone marrow in 5 cases (21%), granulocyte colony-stimulating factor–mobilized peripheral blood stem cell (PBSCs) in 18 cases (75%), and double umbilical cord blood in 1 case. Thirteen patients (54%) received a reduced-intensity conditioning regimen, and 11 patients (46%) received a myeloablative conditioning regimen. Eight patients (33%) received in vivo T cell depletion by using antithymocyte globulin. Four patients received rituximab before allo-SCT to treat their underlying malignancy (2 with chronic lymphocytic leukemia and 2 with follicular lymphoma). For GVHD prophylaxis, patients received CsA either alone (n = 4; 17%) or in combination with methotrexate (n = 16; 67%) or mycophenolate mofetil (n = 4; 17%).
Baseline cGVHD characteristics
cGVHD assessment at baseline is summarized in Table 2. The median time from allo-SCT to cGVHD diagnosis was 5.9 months (range, 2.4-32.7 months), and patients were included in the study at a median of 6.7 months (range, 4.2-33.6 months) after allo-SCT. All patients but 2 had overall, severe (n = 15; 62%) or moderate (n = 7; 29%) cGVHD NIH scores. The 2 (8%) patients with mild cGVHD included 1 patient with mild joint and fascia involvement and 1 patient with mild mouth and eye involvement; both patients required systemic immunosuppressive therapy according to the physician in charge. A total of 92% of patients (n = 22) had ≥2 sites involved at diagnosis, including 8 (33%) with ≥5 sites involved. Ten patients (42%) presented with de novo cGVHD, whereas cGVHD occurred after resolute acute GVHD in 14 patients (58%). Of note, 7 patients still received primary immune suppression with CsA at the time of cGVHD diagnosis and study inclusion. Two patients received CSs alone (n = 1) or in combination with CsA on a taper from prior acute GVHD therapy.
Safety assessment
Forty courses of rituximab in combination with CsA and CSs were given to 24 patients, all of whom were evaluable for toxicity assessment. Rituximab was started at a median of 7 days (range, 1-14 days) after patient inclusion. None of the patients were in response before rituximab initiation. Eight patients received only 1 course of rituximab because of CR achievement (n = 5), resistance (n = 2), or study withdrawal related to cGVHD progression under treatment (n = 1). Overall, a total of 11 nonhematologic, study-related serious adverse events were reported. Most events were infectious in nature and included 3 cases of sepsis, 2 lung infections, 1 sinus infection, and 1 case of progressive multifocal leukoencephalopathy (PML) related to a documented John Cunningham (JC) virus infection. Noninfectious severe adverse events included 1 case of hyperglycemia, 1 case of acute renal failure, 1 case of thrombotic microangiopathy, and 1 case of transient global amnesia. All nonhematologic serious adverse events but PML resolved without sequels. PML was diagnosed in a patient with acute myeloid leukemia who received a myeloablative (cyclophosphamide + 12 Gray total body irradiation) allo-SCT from a matched related donor. This patient received GVHD prophylaxis by methotrexate and CsA and developed de novo severe cGVHD at day 175 after allo-SCT. PML was diagnosed 321 days after inclusion and administration of 2 courses of rituximab and caused his death. Regarding hematologic adverse events, grade 3-4 lymphopenia was reported in 12 patients (50%), and grade 3-4 neutropenia was reported in 5 patients (21%). In 3 patients, neutropenia was clearly related to other causes (underlying disease relapse, CMV reactivation treated by preemptive gancyclovir/valganciclovir, and GVHD), whereas in the other 2 patients, delayed onset neutropenia after rituximab administration could not be excluded.
Clinical response and prednisone taper
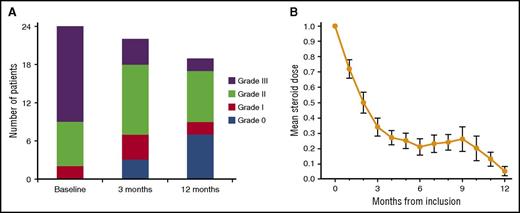
The overall response rate at 1 year after the first rituximab administration was 83%, including 7 patients with CR (29%) and 13 with PR (54%). Treatment failures, as defined per protocol, were reported in 2 patients (8%). One patient’s cGVHD progressed under treatment and the patient was withdrawn from the study at day 49 after the first cycle of rituximab. The second case of treatment failure was a patient who did not respond to the first cycle of rituximab, received additional immunosuppressive therapy with total lymphoid irradiation, and who died of sepsis at day 120. Furthermore, 2 patients in PR after the first and second cycle of rituximab lost their responses. This occurred in 1 patient after 6 months, who remained stable until the last follow-up at 1 year; the second patient lost his response at 3 months, and he died of infection at day 95. Regarding the 5 patients who achieved CR after the first cycle of rituximab, 3 patients remained in CR, whereas 2 patients lost their CR and remained in PR until the last follow-up. In addition, 1 of the patients who was initially not responding after the first course of rituximab and did not receive a second course finally achieved a PR at 3 months without further immunosuppressive therapy and remained in PR until the last follow-up. Regarding the patients in PR after the first cycle of rituximab and who received a second cycle of rituximab, 4 patients converted from PR to stable CR until the last follow-up, whereas 10 patients stayed in PR. We did not observe any flare of cGVHD after rituximab exposure. Two additional patients died during study follow-up, 1 at day 320 from underlying disease relapse, and 1 at day 104 from infection. Both patients were in PR at last follow-up and were considered so in the overall response rate, as defined per protocol. The estimated 1-year OS was 83%, and the 1-year cumulative incidence of NRM was 14%. Evolution of cGVHD NIH score is indicated in Figure 2A.
For the 23 evaluable patients at 3 months after the first infusion of rituximab, CS was decreased by ≥30% in 19 patients (83%) and withdrawn in 2 patients (9%). At 12 months, among 19 evaluable patients (one study withdrawn and 4 dead), 14 (74%) were off CS, and CS had been reduced ≥30% in 4 patients (21%). The mean dose of CS administered during the study follow-up is indicated in Figure 2B.
Evolution in cGVHD NIH score and changes in CS dose. (A) cGVHD NIH score at baseline, at 3 months, and at 12 months. (B) Mean dose of prednisone-equivalent CS in milligrams per kilograms per day with standard error of the mean.
Evolution in cGVHD NIH score and changes in CS dose. (A) cGVHD NIH score at baseline, at 3 months, and at 12 months. (B) Mean dose of prednisone-equivalent CS in milligrams per kilograms per day with standard error of the mean.
QOL assessment
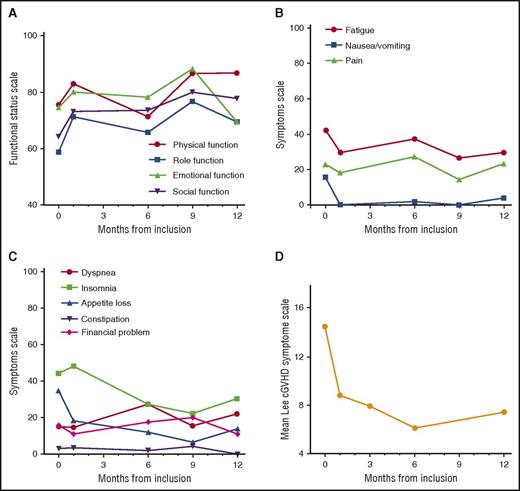
The evolution of QOL, assessed by using the EORTC QLQ-C30 questionnaire25 and the Lee Chronic GVHD Symptom Scale,26 is represented in Figure 3A-D. No worsening of QOL was reported for any of the parameters under treatment. In fact, there was moderate improvement of the functional status scales (physical, role, emotional, and social functioning; Figure 3A), and of the symptom scale, in particular for fatigue, insomnia, nausea/vomiting, and appetite loss (Figure 3B-C). Finally, improvement in the Lee Chronic GVHD Symptom Scale26 scores were reported immediately after the first cycle of rituximab (Figure 3D).
QOL assessment. (A) Mean functional status scale score according to the EORTC QLC-C30 (physical, role, emotional, and social functioning); higher scores on the combined global health status/QOL and functional scale represent a better level of functioning. (B-C) Mean symptom scale scores according to the EORTC QLC-C30 (fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss, diarrhea, constipation, and financial impact); higher scores on symptom scale correspond to a higher level of symptoms. (D) Mean Lee Chronic GVHD Symptom Scale scores.
QOL assessment. (A) Mean functional status scale score according to the EORTC QLC-C30 (physical, role, emotional, and social functioning); higher scores on the combined global health status/QOL and functional scale represent a better level of functioning. (B-C) Mean symptom scale scores according to the EORTC QLC-C30 (fatigue, nausea/vomiting, pain, dyspnea, insomnia, appetite loss, diarrhea, constipation, and financial impact); higher scores on symptom scale correspond to a higher level of symptoms. (D) Mean Lee Chronic GVHD Symptom Scale scores.
PDL1hi B cells are decreased in patients with cGVHD and increased after anti-CD20 rituximab treatment
We then sought to assess a recently described population of rituximab-resistant PD-L1hi B cells that are critical regulators of humoral immunity mediated by Tfh cells.27 PD-L1 expression was assessed within the commonly recognized B-cell subsets in peripheral blood28 : CD19+CD27–IgD+CD38hiCD24hi transitional, CD19+CD27–IgD+CD38intCD24int/low naive, CD19+CD27+IgD+ marginal zone–like, CD19+CD27+IgD– switched memory B cells, and CD19+CD38hiCD24– plasmablasts.
CD19+ B cells were compartmentalized into 3 distinct populations based on PD-L1 expression (PD-L1lo, PD-L1int, and PD-L1hi) (Figure 4A). In healthy donors, the majority of PD-L1hi cells were enriched within the naive compartment (Figure 4B), as reported by Khan et al.27
PDL1hiB cells are decreased in patients with cGVHD and increased after anti-CD20 rituximab treatment. (A) PD-L1 expression on CD19+ live peripheral blood mononuclear cells yields 3 distinct populations (PD-L1lo, green; PD-L1int, blue; and PD-L1hi, red); the number adjacent to the gates defines the percentage of total live CD19+ cells. The graph depicts median fluorescence intensities (MFI) of PD-L1 on CD19+ cells. Data are representative of 16 patients (median, range). (B) CD19+ PD-L1hi cells as a proportion of total live CD19+ cells (mean, standard error of the mean). (C) Naive CD19+ B cells as a percentage of total live CD19+ cells in healthy donors (HD) in allo-SCT recipients with no cGVHD, at diagnosis of cGVHD and at 12 months after the diagnosis of cGVHD and rituximab treatment (mean, standard error of the mean). (D) Absolute values of naive CD19+ B cells (mean, standard error of the mean). (E) PD-L1hi cells as a percentage of total live naive CD19+ B cells (mean, standard error of the mean). (F) Absolute values of PD-L1hi naive CD19+ B cells (mean, standard error of the mean). MZ-like, marginal zone-like; ns, not significant.
PDL1hiB cells are decreased in patients with cGVHD and increased after anti-CD20 rituximab treatment. (A) PD-L1 expression on CD19+ live peripheral blood mononuclear cells yields 3 distinct populations (PD-L1lo, green; PD-L1int, blue; and PD-L1hi, red); the number adjacent to the gates defines the percentage of total live CD19+ cells. The graph depicts median fluorescence intensities (MFI) of PD-L1 on CD19+ cells. Data are representative of 16 patients (median, range). (B) CD19+ PD-L1hi cells as a proportion of total live CD19+ cells (mean, standard error of the mean). (C) Naive CD19+ B cells as a percentage of total live CD19+ cells in healthy donors (HD) in allo-SCT recipients with no cGVHD, at diagnosis of cGVHD and at 12 months after the diagnosis of cGVHD and rituximab treatment (mean, standard error of the mean). (D) Absolute values of naive CD19+ B cells (mean, standard error of the mean). (E) PD-L1hi cells as a percentage of total live naive CD19+ B cells (mean, standard error of the mean). (F) Absolute values of PD-L1hi naive CD19+ B cells (mean, standard error of the mean). MZ-like, marginal zone-like; ns, not significant.
As expected, although CD27– transitional and naive B cells were significantly increased after allo-SCT compared with healthy donors, antigen experienced CD27+ marginal zone–like and switched memory B cells were significantly decreased (Figure 4C-D; supplemental Figure 1, supplemental Table 2). However, there was no difference in the B-cell compartments between patients without cGVHD and patients with newly diagnosed cGVHD (Figure 4C-D; supplemental Figure 1).
We next investigated the presence of PDL1hi B cells after allo-SCT in patients with and without cGVHD. At diagnosis of cGVHD, patients had a significantly lower percentage of PDL1hi naive B cells compared with allo-SCT patients without cGVHD (2.4% vs 6.9%, P = .007; Figure 4E). In contrast, although, as expected, the number of CD19+ B cells significantly decreased after anti-CD20 rituximab treatment (8.0 × 106/L vs 251.9 × 106/L; P = .002; supplemental Figure 1B), the percentage of PDL1hi B cells was significantly increased (6.7% vs 2.4%, P = .01). Therefore, despite being significantly lower in absolute values (Figure 4F), the percentage of PDL1hi naive B cells was significantly higher 12 months after anti-CD20 rituximab treatment (Figure 4E).
Circulating Tfh cells are decreased after anti-CD20 rituximab treatment
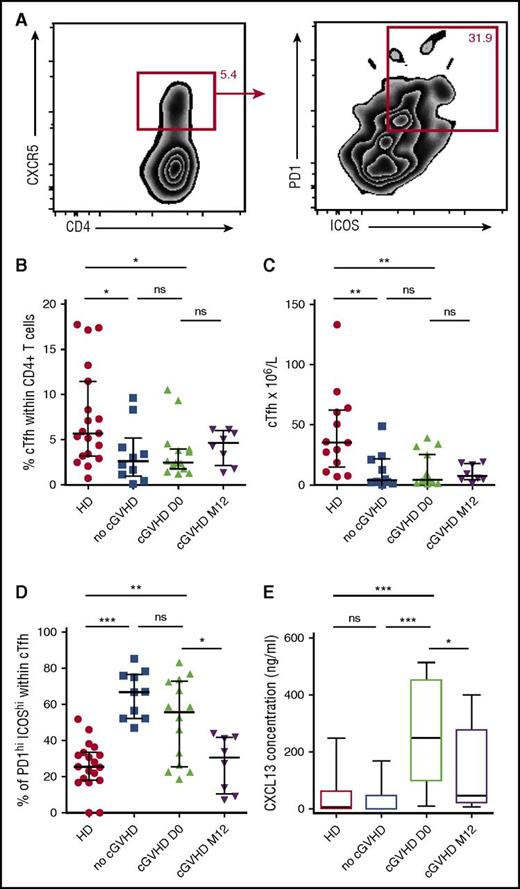
Given the role of PDL1hi B cells in regulating humoral immunity mediated by Tfh cells,27 we sought to assess CD4+CD45RO+CXCR5+ circulating Tfh (cTfh) cells in those patients (Figure 5A). cTfh cells were significantly decreased after allo-SCT both in patients with and without cGVHD, with no difference in percentages and absolute numbers between the 2 groups (2.5% vs 2.6%; P = .56 and 4.3 × 106/L vs 3.8 × 106/L; P = .64; Figure 5B-C). Given that functionally active Tfh cells express high levels of ICOS and PD-1, we decided to focus on activated cTfh ICOShi PD-1hi cells (Figure 5A). After allo-SCT, we observed a significant increase in the percentage of activated cTfh cells both in patients with and without cGVHD compared with healthy donors (55.6% and 66.8% vs 25.5%; P < .0001 and P = .004; Figure 5D). However, after anti-CD20 therapy, there was a significant decrease of activated cTfh cells (30.6% vs 55.6%; P = .03), which went back to the levels found in healthy donors (30.6% vs 25.5%; P = .77).
Circulating Tfh cells are decreased after anti-CD20 rituximab treatment. (A) ICOShi PD-1hi expression on live circulating Tfh CD45+CD3+CD4+CD45RO+CXCR5+ cells. (B) Percentage of circulating Tfh cells among total live CD4+ cells in healthy donors (HD) and in allo-SCT recipients with no cGVHD, at diagnosis of cGVHD, and 12 months after the diagnosis of cGVHD and rituximab treatment (mean, standard error of the mean). (C) Absolute values of circulating Tfh cells. (D) Percentage of ICOShi PD-1hi cells among total live circulating Tfh cells (mean, standard error of the mean). (E) Whisker plots represent plasma CXCL13 concentrations in healthy donors and in each patient group after allo-SCT. HD, healthy donors.
Circulating Tfh cells are decreased after anti-CD20 rituximab treatment. (A) ICOShi PD-1hi expression on live circulating Tfh CD45+CD3+CD4+CD45RO+CXCR5+ cells. (B) Percentage of circulating Tfh cells among total live CD4+ cells in healthy donors (HD) and in allo-SCT recipients with no cGVHD, at diagnosis of cGVHD, and 12 months after the diagnosis of cGVHD and rituximab treatment (mean, standard error of the mean). (C) Absolute values of circulating Tfh cells. (D) Percentage of ICOShi PD-1hi cells among total live circulating Tfh cells (mean, standard error of the mean). (E) Whisker plots represent plasma CXCL13 concentrations in healthy donors and in each patient group after allo-SCT. HD, healthy donors.
Finally, we decided to assess the levels of the B-cell–attracting chemokine CXCL13 in our cohort of patients. The CXCL13 receptor is CXCR5, which is also expressed by Tfh cells, therefore leading to the attraction of both B cells and Tfh cells to the lymphoid follicle. Although there was no difference in CXCL13 levels between healthy donors and patients without cGVHD, it was significantly increased at diagnosis of cGVHD as compared with the levels found in patients without cGVHD (249.6 pg/mL vs 0.5 pg/mL; P = .0005; Figure 5E). At 1 year after cGVHD treatment, CXCL13 decreased significantly from 249.6 pg/mL to 45.7 pg/mL (P = .04).
Discussion
The addition of rituximab to standard first-line therapy for cGVHD evaluated in the current phase 2 prospective study has successfully fulfilled its objective with an overall response rate at 1 year of 83%. Solomon et al22 reported a similar overall response rate of 88% using calcineurin inhibitor and rituximab without CSs, but that treatment was associated with a high recurrence of cGVHD, estimated as 20% at 1 year and 37% at 2 years. In contrast, in our study, the recurrence rate at 1 year was only 9%, therefore maintaining CSs as part of first-line treatment of cGVHD, including in patients receiving rituximab, seems to be associated with better long-term control of cGVHD. One could object that long-term use of CSs is associated with a large range of side effects, including impaired immune function and an increased risk of opportunistic infections. However, our therapeutic strategy was associated with early steroid withdrawn, with 74% of patients off CSs at 1 year. This achievement is particularly relevant, because lower steroid doses at 12 months after initial treatment are associated with long-term success of withdrawing all immunosuppressive treatment.29 In addition, in our study, two-thirds of patients received a second course of rituximab, leading to an improvement from PR to CR in 4 of them (4 of 16 patients, 25%). So far, no previous studies have demonstrated any benefit of the addition of other agents (eg, mycophenolate mofetil, thalidomide, or hydroxychloroquine) to prednisone and inhibitor of calcineurin.5,6,30,31 In particular, no improvement of CS withdrawal was reported in those studies. Our results suggest that the addition of rituximab may be effective; however, randomized prospective studies are warranted to confirm these findings.
Rituximab-based, first-line treatment of cGVHD was well tolerated, with only 11 nonhematologic serious adverse events, including 7 infections. All adverse events but PML resolved without sequelae. This incidence of infectious complications is very low for patients under immunosuppressive therapy, highlighting that the addition of rituximab not only was not associated with an increased risk of infections but, on the contrary, decreased that risk in patients with cGVHD by accelerating CS withdrawal. Similarly, only 1 patient relapsed during study follow-up, suggesting that enhanced immunosuppressive therapy with the addition of rituximab was not associated with a decreased graft-versus-tumor effect. However, we must highlight that 1 patient presented with fatal PML, an exceptional life-threatening casualty associated with the use of rituximab.32 Besides having received an allo-SCT, this patient had no risk factors other than the administration of 2 courses of rituximab, raising the issue of cumulative exposure to rituximab in PML onset. Indeed, no PML cases have been reported after the administration of 4 infusions of rituximab for cGVHD prophylaxis.20,21 However, in the largest report of PML after rituximab administration in patients with hematologic malignancies,32 the median number of doses of rituximab infusion was 6 (range, 1-28) showing that PML could develop after low doses of rituximab and that the cumulative incidence of rituximab exposure is not the only driver of PML onset. Overall, with regard to the available data, one must remain cautious with prolonged exposure to rituximab after allo-SCT. With respect to grade 3-4 neutropenia, the diagnosis of delayed onset neutropenia after rituximab administration could be retained in 2 patients, which is in line with the rate reported in the literature (5%-15%).33 Of note, none of these patients presented with infectious complications related to neutropenia. Overall, our therapeutic strategy appears to be safe, with a low NRM rate of 14% and an OS rate of 83% at 1 year. Furthermore, we note that patients who failed to respond to rituximab (eg, treatment failure or loss of response) had worse outcomes, with a significant mortality, that may be related to the use of higher doses of steroids and of additional immunosuppressive treatments in those patients. Therefore, particular attention must be paid to those patients, and it is of significant interest to identify biomarkers that can predict response to improve cGVHD management.
A comprehensive assessment of QOL was also included in our study. All parameters of the EORTC QLQ-C30 remained stable or improved right after the first cycle of rituximab, in particular for fatigue, insomnia, nausea/vomiting, and appetite loss. Similarly, the Lee Chronic GVHD Symptom Scale scores improved after the first cycle of rituximab, highlighting that our therapeutic strategy was associated with global improvement of the symptoms of cGVHD from the patient’s point of view.
Based on our findings, targeting B cells appears to be an effective therapeutic strategy for first-line treatment of cGVHD in accordance with the growing amount of data highlighting the role of B cells in cGVHD pathophysiology.10-12,34 Therefore, it was recently reported that IL-10–producing regulatory B cells (Bregs) probably play a role in the prevention of cGVHD because these cells have been reported to be decreased in patients with active cGVHD.35,36 However, although Bregs can mediate immune suppression independently of IL-10,37,38 the role of these subsets has never been explored in the setting of cGVHD. In particular, PD-L1hi B cells have been reported to be critical regulators of humoral immunity mediated by Tfh cells, which have been recently reported to be involved in cGVHD pathophysiology, both in mouse models39 and in humans.40
We confirmed that although PD-L1 expression was not confined to a single B-cell subset, PD-L1hi B cells were more frequent within the naive CD27–IgD+CD38intCD24int/low B-cell compartment. This is particularly interesting given that naive and transitional B cells increase after allo-SCT, although antigen-experienced B cells are decreased. Furthermore, the increase in naive B cells is accompanied by an increase in PD-L1hi B cells in patients without cGVHD. Similar to IL-10–producing Bregs, we reported a decrease in PD-L1hi naive B cells in patients with cGVHD compared with patients without it, suggesting that the expansion of those cells after allo-SCT plays a role in controlling cGVHD.
Given that PDL1hi B cells can regulate humoral immunity mediated by Tfh cells, we assessed CD45RO+CXCR5+ cTfh cells in those patients to decipher how PD-L1hi naive B cells can control cGVHD. Although the cTfh cells were significantly decreased after allo-SCT, the percentage of activated cTfh cells significantly increased after allo-SCT. Of note, there was no difference in cTfh cells, either total or activated, between patients with and without cGVHD. However, we cannot exclude a role for Tfh cells in cGVHD given that CXCL13, a chemokine that attracts both B cells and Tfh cells to the lymphoid follicle, is significantly increased in patients with cGVHD, suggesting an increased homing of Tfh cells to the secondary lymphoid organ. These findings are in agreement with the study of Flynn et al,39 which showed, in a murine model, that trafficking of CXCR5+ T cells to lymphoid follicles supports cGVHD development and that cGVHD is associated with an increased percentage of Tfh cells within lymphoid follicles. In contrast with our findings, Forcade et al40 reported that patients with active cGVHD have a decreased number of cTfh cells compared with patients without cGVHD. However, in that study, any patient with active cGVHD could be included, including those on CSs, whereas, in our study, all patients were included at diagnosis of cGVHD before any CS administration. Therefore, the decreased level of cTfh reported by Forcade et al40 may be related to the CS treatment administered in those patients. Our hypothesis was strengthened by our findings 1 year after rituximab treatment. Activated cTfh cells and CXCL13 significantly decreased, whereas PDL1hi B cells increased, suggesting that although Tfh cells are no longer attracted in the lymphoid follicles by CXCL13, increased PDL1hi B cells may contribute to control activated Tfh cells, leading to their decrease. Of note, with respect to CXCL13, Forcade et al40 found a similar increase in patients with active cGVHD and a decrease in patients with resolved cGVHD, suggesting that this kinetic is not related to rituximab but to cGVHD response. Because samples were available for only 16 patients receiving rituximab, and all of them were in response, no comparison could be performed on B and Tfh cell subsets between patients with and without response.
In conclusion, our study shows that the addition of rituximab to CSs and CsA is a safe and effective first-line treatment of cGVHD. In addition, we contributed to improving the knowledge of cGVHD pathophysiology by providing the framework for a possible new mechanism of action of rituximab in the setting of cGVHD, mediated through PDL1hi B cells and Tfh cells. This hypothesis remains to be confirmed in a randomized, prospective phase 3 clinical trial evaluating CSs and CsA with or without rituximab for cGVHD first-line treatment. Caution is required for the dose of rituximab (1 or 2 cycles) to be used in such setting in regards to the risk of PML in those patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank V. Dehame for technical and logistical support. The authors also thank the nursing staff for providing excellent care for our patients. M.M. thanks J.V. Melo for critical reading of the manuscript.
This work was supported by a grant from the French Ministry of Health and the Institut National du Cancer as part of the Programme Hospitalier de Recherche Clinique. This work was also supported by the Association for Training, Education, and Research in Hematology, Immunology, and Transplantation. This work was carried out in the context of the Institut Hospitalo-Universitaire-Cesti project, which received French government financial support managed by the National Research Agency via the investment of the future program ANR-10-IBHU-005. The Institut Hospitalo-Universitaire-Cesti project is also supported by Nantes Metropole and the Pays de la Loire Region.
Roche France provided the drug Rituximab studied in this work, but did not participate to protocol design, data analysis, or manuscript writing.
Authorship
Contribution: M.M., F.M, and B.G. contributed to study conception and design; M.M. contributed to financial support; M.M., B.V., and G.B. provided administrative and logistical support; T.G., I.Y.-A., S.C., P.C., D.B., R.T., L.M., P.M., and M.M. provided study materials and patient care; F.M. contributed to the experimental work; F.M. and M.M. contributed to the collection and assembly of clinical data; F.M., M.L., B.G., and M.M. contributed to data analysis and interpretation; F.M., B.G., and M.M. wrote the manuscript; and all authors contributed substantially to this work and gave final approval of manuscript.
Conflict-of-interest disclosure: M.M. received research support and lectures honoraria from Roche, whose drug is studied in this work. The remaining authors declare no competing financial interests.
Correspondence: Florent Malard, Centre de Recherche en Transplantation et Immunologie, Unité Mixte de Recherche 1064, Institut National de la Santé et de la Recherche Médicale, Université de Nantes, 30 Bd Jean Monnet, F-44093 Nantes Cedex, France; e-mail: florent.malard@inserm.fr; and Mohamad Mohty, Service d’Hématologie Clinique et Thérapie Cellulaire, Hôpital Saint-Antoine, 75012 Paris, France; e-mail: mohamad.mohty@inserm.fr.