Key Points
VLITL is amyloid prone and forms the ends of Aα-chain fibrils in vivo.
VLITL explains the molecular basis of Aα-chain amyloidogenesis.
Abstract
The first case of hereditary fibrinogen Aα-chain amyloidosis was recognized >20 years ago, but disease mechanisms still remain unknown. Here we report detailed clinical and proteomics studies of a French kindred with a novel amyloidogenic fibrinogen Aα-chain frameshift variant, Phe521Leufs, causing a severe familial form of renal amyloidosis. Next, we focused our investigations to elucidate the molecular basis that render this Aα-chain variant amyloidogenic. We show that a 49-mer peptide derived from the C-terminal part of the Phe521Leufs chain is deposited as fibrils in the patient’s kidneys, establishing that only a small portion of Phe521Leufs directly contributes to amyloid formation in vivo. In silico analysis indicated that this 49-mer Aα-chain peptide contained a motif (VLITL), with a high intrinsic propensity for β-aggregation at residues 44 to 48 of human renal fibrils. To experimentally verify the amyloid propensity of VLITL, we generated synthetic Phe521Leufs-derived peptides and compared their capacity for fibril formation in vitro with that of their VLITL-deleted counterparts. We show that VLITL forms typical amyloid fibrils in vitro and is a major signal for cross-β-sheet self-association of the 49-mer Phe521Leufs peptide identified in vivo, whereas its absence abrogates fibril formation. This study provides compelling evidence that VLITL confers amyloidogenic properties to Aα-chain frameshift variants, yielding a previously unknown molecular basis for the pathogenesis of Aα-chain amyloidosis.
Introduction
Fibrinogen is a 340-kDa glycoprotein, composed of 2 identical heterotrimers, each consisting of 1 Aα, 1 Bβ, and 1 γ-chain.1 Mutations altering any of these 3 chains are commonly associated with autosomal or recessive bleeding/thrombotic disorders (http://site.geht.org/base-fibrinogene/) without any clinical evidence of amyloidosis. However, a small fraction of Aα-chain variants are amyloidogenic and lead to massive Aα-chain deposition as amyloid fibrils in a fibrinogen Aα-chain-derived amyloidosis (AFib) patient’s kidneys. The first case of AFib has been described by Benson in 1993,2 and until now, only the Aα-chain has been linked to AFib (http://amyloidosismutations.com/mut-afib.php). AFib is a rare, late-onset, autosomal dominant condition characterized by massive amyloid deposition in the glomerular compartment of the kidney.2 Heterozygous AFib patients typically display a chronic kidney disease in the fourth/fifth decade of life leading to progressive end-stage renal failure.3 Although amyloid mechanisms involved in AFib are still unknown, it was shown that AFib fibrils were exclusively composed of the mutant Aα-chain,2,4 suggesting that the wild-type Aα-chain does not contribute to amyloid deposition, analogous to what was previously observed in patients with familial lysozyme, β2-microglobulin, and apoC-III amyloidosis.5-7 The therapeutic approach of AFib consists only of supportive treatment by dialysis and by renal or combined hepatorenal transplantation. Recently, Benson’s group suggested that preemptive hepatic transplantation may avert the progression of renal damage and may be a promising treatment of AFib prior to the need for renal dialysis or kidney transplantation.8 Although the first case of AFib was recognized >20 years ago, we still do not know which specific part of mutant Aα-chain sequences directly participates in the β-aggregation process. To date, a total of 15 Aα-chain variants are known to be amyloid prone in humans, and remarkably, these amyloidogenic variants are all clustered in a small portion of the Aα-chain from residues 517 to 555.2-4,9-13 Missense Aα-chain variants have been reported in several AFib families worldwide, with Glu526Val being the most common amyloidogenic Aα-chain variant.9 In contrast, amyloidogenic Aα-chain frameshift variants are “private” (ie, each of them has been reported in only a single family), and therefore, available clinical information associated with these variants is very limited.3,4,10,12,13 Two of them have exceptionally been associated with pediatric AFib cases,4,10 suggesting that frameshifts may be particularly aggressive and are likely highly prone to self-aggregation; therefore, it is of particular interest to investigate the precise mechanisms responsible for their amyloidogenicity.
We report a novel “private” amyloidogenic Aα-chain frameshift variant (c.1620delT/Phe521Leufs) and show that renal fibrils of AFib patients are composed of a short polypeptide derived from the C-terminal part of the Phe521Leufs Aα-chain without evidence of a wild-type counterpart. This additional confirmation of what part of the mutant Aα-chain is deposited in the disease tissue of AFib patients prompted us to explore how the mutant Phe521Leufs chain contributes to Aα-chain amyloid formation.
Materials and methods
Genetic analysis
Blood samples from the family members were obtained after their written informed consent. This study had the approval of the Ethics Committee of the Hospital of Rennes and was performed according to the Declaration of Helsinki. The entire coding region and flanking splice sites of exon 5 of the fibrinogen alpha gene (FGA; MIM_134820) were sequenced as previously described.4 The nomenclature of the fibrinogen Aα-chain deletion is based on the FGA transcript reference (NM_000508.3). For more clarity with the historically conventional nomenclature, the FGA variant is described as Phe521Leufs according to the mature protein without the signal peptide. According to the recommended Human Genome Variation Society (HGVS), which starts the amino acid numbering at the initiator methionine, Phe521Leufs corresponds to Phe540Leufs. In Table 1, all amyloidogenic Aα-chain frameshift variants are listed with the 2 nomenclatures. To convert the conventional mature protein amino acid numbering to the HGVS nomenclature, add 19 nucleotides for Aα-chain changes.
Histology and transmission electron microscopy (TEM) of renal biopsies
Laser microdissection/liquid chromatography and tandem mass spectrometry (LMD/MS) analysis
The LMD/MS methods have been previously summarized in full text.14
In silico tools
AMYLPRED2 is a consensus algorithm for prediction of amyloidogenic determinants combining 11 different methods (available at http://biophysics.biol.uoa.gr/AMYLPRED2/).15 The cross-β TANGO score results from a statistical mechanics model based on simple physico-chemical principles of secondary structure formation.16 The PASTA energy is indicative of the aggregation propensity and predicts which portions of the sequence are more likely to stabilize the cross-β core of fibrillar aggregates.17
Fibril formation
All 5 synthetic peptides were purchased from Genepep (France), and stock solutions were prepared at the final concentration of 2 to 6 mM and stored at –20°C. Fibril formation was induced in 10 mM 4-morpholinepropanesulfonic acid buffer, 150 mM NaCl, pH 7.2 at a final peptide concentration ranging from 0.125 to 1 mM. Peptide solutions were incubated at room temperature without agitation or adjuvants from 1 day to 2 months.
Fluorescence experiments
One hundred microliters of the 5 peptides was added to 900 µL of fibrillation buffer containing Thioflavin T (ThT; 5 µM final concentration). Fluorescence was measured with a Perkin-Elmer Luminescence Spectrometer LS55 with slit widths of 10 nm. The excitation was at 450 nm, and emission spectra of ThT were recorded from 470 to 600 nm.
TEM of aggregates formed in vitro
Aliquots of the 5 peptides (10 µL with a concentration of ∼10 mg/mL) were placed on carbon-coated copper grids (300 mesh), washed, negatively stained for 1 minute with 1% (weight-to-volume ratio) uranyl acetate, and wicked dry prior to analysis using a Philips CM12 transmission electron microscope operating at accelerating voltages of 120 kV.
X-ray microdiffraction (XRD) experiments performed on in vitro aggregate samples and on ex vivo renal fibrils
All samples were pelleted by centrifugation at 5000g for 10 minutes. Small concentrated drops of samples were deposited on cylindical fibers of ∼100 µm diameter. XRD was performed at the European Synchrotron Radiation Facility (Grenoble, France) on the microfocus beam line ID13, as previously described.18
Results
Phe521Leufs is a novel amyloidogenic “private” frameshift variant of fibrinogen Aα-chain associated with a severe form of renal amyloidosis
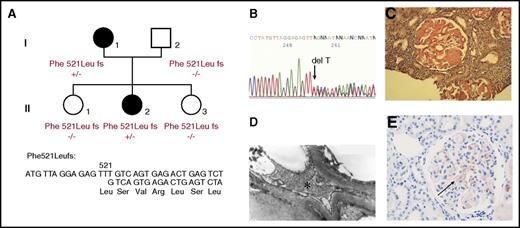
Amyloidosis was initially diagnosed from a 27-year-old woman (patient I.1) who presented proteinuria (1.30 g/L) during routine medical screening at her first pregnancy (Figure 1A). Progressively, she developed nephrotic syndrome without hypertension (120/80 mm Hg). On physical examination, she had no signs of peripheral or autonomic neuropathy, and all cardiac investigations were normal. A renal biopsy was performed, and amyloidosis was diagnosed, but the etiology of her renal amyloidosis remained undetermined. Three years later, this patient was diagnosed with malignant hypertension (210/130 mm Hg) and was treated with transfusion of fresh frozen plasma and association of several antihypertensive drugs, resulting in adequate control of blood pressure. However, she continued to develop uremia leading to acute renal failure requiring hemodialysis. One year later, she underwent her first renal transplantation, but amyloidosis recurred on the renal graft 5 years later, and she received her second renal graft. The etiology of this renal amyloidosis was reevaluated when one of her daughters (II.2) began to manifest proteinuria at 22 years old associated with glomerular amyloid deposits. This proband’s daughter also developed an acute episode of malignant hypertension leading to acute renal failure and hemodialysis. In both of these affected AFib probands, there was no history of bleeding or thrombotic disorders (even during surgical procedures and during the nephrotic phase), and all routine coagulation investigations were normal including Clauss fibrinogen activity level, fibrinogen antigen level, activated partial thromboplastin time, prothrombin time, thrombin time, and reptilase time, indicating absence of significant quantitative or qualitative fibrin clot abnormalities. Finally, hereditary AFib was confirmed on the basis of history of renal disease in 2 family members, documented evidence of renal dysfunction secondary to Congo red amyloid deposits in glomeruli, histological evidence of amyloid fibrils at electron microscopy, immunohistochemistry of renal biopsies, and detection of FGA mutation (Figure 1A-E). The 2 AFib probands (I.1 and II.2) were heterozygous for a novel single base pair deletion (c.1620delT), expected to alter the reading frame of the Aα-chain messenger RNA at codon 521: Phe521Leufs (numbering according to the mature protein) or Phe540Leufs (according to the recommended HGVS nomenclature, including the signal peptide). This mutation was not detected in family members II.1 and II.3 who had no proteinuria, confirming that this novel Aα-chain variant segregated with renal disease in this kindred (Figure 1A,E). This thymine deletion is expected to cause loss of the last C-terminal 62 amino acids of the wild-type Aα-chain and, instead, the incorporation of 27 new residues (521-LSVRLSLGAQNLASSQIQRNPVLITLG-547) before premature termination of the translation at codon 548 (Figure 1D and Figure 2B). Therefore, the genetic data were concordant with immunohistochemistry analysis showing that the Congo red glomeruli deposits positively stained with a specific anti-human Aα-chain monoclonal antibody that recognized the mutant C-terminal portion of all amyloidogenic frameshifts (Figure 1E).4 Sequence alignment of Phe521Leufs with amyloidogenic Aα-chain frameshift variants reported thus far showed that all invariably truncate at codon 548, producing highly similar mutant C-terminal sequences with a common portion of 15 amino acids, ASSQIQRNPVLITLG, residues 533 to 547 (Table 1).
Novel amyloidogenic “private” Aα-chain frameshift variant in a French family. (A) The family pedigree of the AFib-kindred with familial segregation of the Phe521Leufs variant because of a single thymine deletion at Phe521. Squares denote male family members; circles, female family members; and solid symbols, affected family members. (B) Partial sequence of FGA exon 5 from II.2, indicating heterozygosity for deletion of a single thymine leading to superimposed sequences after codon 521. (C) Congo red deposits in the glomeruli of renal specimens from II.2 (original magnification ×400). (D) The amyloid fibrils found in the mesangium and under the glomerular basement membrane, appearing as straight unbranched fibrils with 10 nm diameter by electron microscopy (original magnification ×1400). The asterisk indicates the fibrils. (E) Positive staining with the specific Aα-chain antibody raised against the C-terminal Aα-chain mutant sequence (black arrow).
Novel amyloidogenic “private” Aα-chain frameshift variant in a French family. (A) The family pedigree of the AFib-kindred with familial segregation of the Phe521Leufs variant because of a single thymine deletion at Phe521. Squares denote male family members; circles, female family members; and solid symbols, affected family members. (B) Partial sequence of FGA exon 5 from II.2, indicating heterozygosity for deletion of a single thymine leading to superimposed sequences after codon 521. (C) Congo red deposits in the glomeruli of renal specimens from II.2 (original magnification ×400). (D) The amyloid fibrils found in the mesangium and under the glomerular basement membrane, appearing as straight unbranched fibrils with 10 nm diameter by electron microscopy (original magnification ×1400). The asterisk indicates the fibrils. (E) Positive staining with the specific Aα-chain antibody raised against the C-terminal Aα-chain mutant sequence (black arrow).
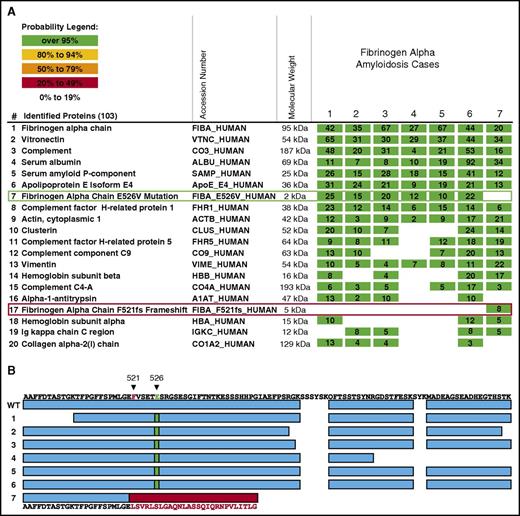
The C-terminal mutant region predicted by Phe521Leufs is detected in renal amyloid deposits, but not its wild-type counterpart. (A) The results of LMD/MS-based proteomics analysis of amyloid plaques from 7 cases of AFib. Cases 1 to 6 carry the Aα-chain Glu526Val (E526V) variant, and case 7 the Phe521Leufs (F521fs) variant. The identified proteins are listed by relative probability score for identity, and the top 20 proteins of 103 proteins are shown. The columns show the protein name, the UnitProt identifier (protein accession number in the UniProt database, http://www.uniprot.org/), the molecular weight of the protein, and 1 microdissection from 7 patient specimens involved by AFib. The numbers indicate the number of total peptide spectra identified for each protein. Fibrinogen Aα-chain is the most abundant protein amyloidogenic in this sample set, consistent with AFib amyloidosis in each case. To show the presence of mutated proteins in the amyloid plaques, the raw mass spectrometry data files were searched using the human SwissProt database supplemented with Aα-chain variants Glu526Val and Phe521Leufs. Consistent with genetic analysis, cases 1 to 6 contain the tryptic peptide carrying the Glu526Val variant (row 7, green rectangle), whereas this variant is not present in the case with the Phe521Leufs variant. In contrast, the novel tryptic peptides generated by the frameshift in Phe521Leufs variant are only present in case 7 (row 17, red rectangle). (B) shows the Aα-chain protein coverage in 7 cases of AFib amyloidosis. Cases 1 to 6 carry the Glu526Val variant, and case 7 the Phe521Leufs variant. The top line represents the C-terminal sequence of native Aα-chain. The amino acid residues of Phe521Leufs (F521 in red) and Glu526Val (E526 in green) are indicated. The first line of rectangles (blue) labeled “WT” represents the coverage of the wild-type Aα-chain by the mass spectrometry-based proteomic method used. Two samples (S1 and S2) from 4 patients are shown. In cases 1 to 6 (Glu526Val variant), most of the coverage is identical to the wild-type Aα-chain except for the tryptic peptide carrying the point mutation (green rectangle) instead of the amino acid present in the wild-type peptide. In case 7 (Phe521Leufs), frameshift leads to a novel sequence indicated by the red rectangle and amino acid sequence in red letters. No native Aα-chain peptides are present after the frameshift mutation, indicating that only the mutant Aα-chain is present in amyloid deposits.
The C-terminal mutant region predicted by Phe521Leufs is detected in renal amyloid deposits, but not its wild-type counterpart. (A) The results of LMD/MS-based proteomics analysis of amyloid plaques from 7 cases of AFib. Cases 1 to 6 carry the Aα-chain Glu526Val (E526V) variant, and case 7 the Phe521Leufs (F521fs) variant. The identified proteins are listed by relative probability score for identity, and the top 20 proteins of 103 proteins are shown. The columns show the protein name, the UnitProt identifier (protein accession number in the UniProt database, http://www.uniprot.org/), the molecular weight of the protein, and 1 microdissection from 7 patient specimens involved by AFib. The numbers indicate the number of total peptide spectra identified for each protein. Fibrinogen Aα-chain is the most abundant protein amyloidogenic in this sample set, consistent with AFib amyloidosis in each case. To show the presence of mutated proteins in the amyloid plaques, the raw mass spectrometry data files were searched using the human SwissProt database supplemented with Aα-chain variants Glu526Val and Phe521Leufs. Consistent with genetic analysis, cases 1 to 6 contain the tryptic peptide carrying the Glu526Val variant (row 7, green rectangle), whereas this variant is not present in the case with the Phe521Leufs variant. In contrast, the novel tryptic peptides generated by the frameshift in Phe521Leufs variant are only present in case 7 (row 17, red rectangle). (B) shows the Aα-chain protein coverage in 7 cases of AFib amyloidosis. Cases 1 to 6 carry the Glu526Val variant, and case 7 the Phe521Leufs variant. The top line represents the C-terminal sequence of native Aα-chain. The amino acid residues of Phe521Leufs (F521 in red) and Glu526Val (E526 in green) are indicated. The first line of rectangles (blue) labeled “WT” represents the coverage of the wild-type Aα-chain by the mass spectrometry-based proteomic method used. Two samples (S1 and S2) from 4 patients are shown. In cases 1 to 6 (Glu526Val variant), most of the coverage is identical to the wild-type Aα-chain except for the tryptic peptide carrying the point mutation (green rectangle) instead of the amino acid present in the wild-type peptide. In case 7 (Phe521Leufs), frameshift leads to a novel sequence indicated by the red rectangle and amino acid sequence in red letters. No native Aα-chain peptides are present after the frameshift mutation, indicating that only the mutant Aα-chain is present in amyloid deposits.
The C-terminus end of Phe521Leufs chain constitutes amyloid fibrils in vivo
To determine which part of the Aα-chain contributes to the formation of amyloid fibrils, renal deposits from proband II.2 were extracted by LMD/MS,14 and the Phe521Leufs amyloid proteome was compared with the proteome obtained from AFib patients carrying the Glu526Val missense variant, the most common amyloidogenic Aα-chain variant. Several independent samples (replicates) were analyzed for Phe521Leufs and Glu526Val variants. For both variants, the amyloid proteome profile indicated that Aα-chain showed the highest probability score in the deposits and the signature proteins (SAP and apoE) were present, giving confidence in the identification of amyloid (Figure 2A). Peptides corresponding to the new C-terminal sequence of Phe521Leufs were detected only in the case carrying this mutation and not in Glu526Val cases (Figure 2A). Detailed examination of the protein coverage of Phe521Leufs showed that the amyloid peptide contained all of the modified C-terminal sequence encoded by the Phe521Leufs allele (100% coverage) but not the wild-type C-terminal Aα-chain sequence after residue 521 (Figure 2B). In conclusion, the amyloid peptide characterized in Phe521Leufs deposits was a hybrid 49-mer fragment with the first 22 residues identical to the wild-type Aα-chain residues 499 to 520 (AFFDTASTGKTFPGFFSPMLGE) and the C-terminal 27 residues corresponding to the sequence encoded by Phe521Leufs (LSVRLSLGAQNLASSQIQRNPVLITLG) (Figure 2B). Therefore, our amyloid proteome analysis indicated that wild-type Aα-chain does not contribute to amyloid formation, and that only the C-terminal sequence generated by the Phe521Leufs allele is amyloid in vivo, a finding consistent with previous publications.2,4,13
VLITL is predicted to be a major cross-β-sheet signal of Phe521Leufs chain
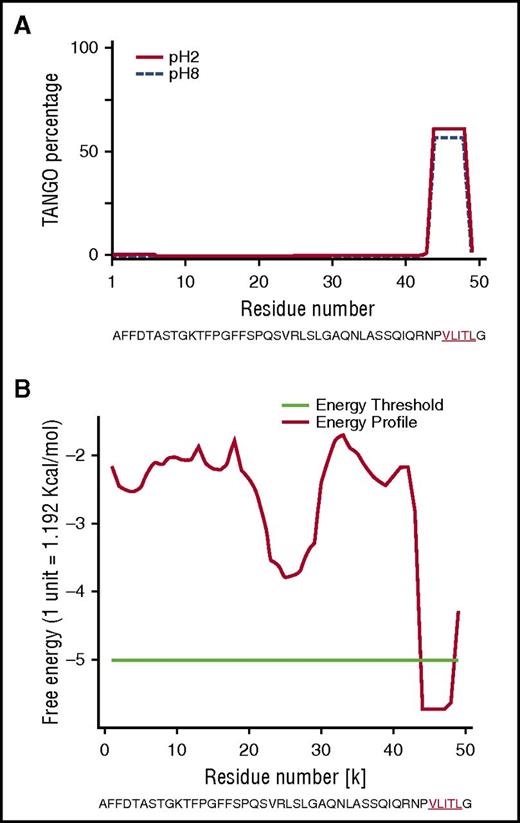
To explore why the mutant Phe521Leufs sequence contributes to amyloid formation, we first performed in silico analyses on the full-length sequences of Phe521Leufs and all frameshifts to search for motifs with a propensity to form amyloid (Table 1).19,20 We used AMYLPRED2,15 which combines 11 individual algorithms, and PASTA2,17 which predicts which amino acid and β-sheet orientation is energetically favored. Combined algorithms indicated that, in all cases, the amino acid sequence encoded by each amyloidogenic frameshift variant created a common short stretch motif with high amyloidogenic propensity that involved a 5-residue fragment (VLITL) (Figure 3A-B). TANGO identified VLITL as the unique hot spot with a high intrinsic propensity for β-aggregation regardless of pH and ionic strength (Figure 3A), and PASTA2 predicted that VLITL forms parallel in-register intermolecular β-sheets in amyloid (favorable pairing energy of −5.57, corresponding to 10.7 kcal/mol) (Figure 3B). Consistent with these predictions, VLITL is consistently present in Aα-chain frameshift variants associated with renal amyloidosis (Table 1), whereas VLITL is absent from the amino acid sequences of those that are not clinically amyloidogenic (http://site.geht.org/base-fibrinogene/).21,22 This genotype-phenotype correlationship suggests that nucleotide indel mutations producing mutant Aα-chains containing VLITL at their C-termini will be likely amyloidogenic (Table 1). More importantly, we show here that VLITL is part of renal fibrils of AFib patient II.2 (residues 44-48 of the 49-mer amyloid peptide found ex vivo) (Figure 2B), reinforcing the view that VLITL indeed might confer amyloidogenic properties.
In silico studies of human Aα-chain amyloidogenic frameshift variants. (A) The TANGO aggregation scores of Phe521Leufs variant exhibited a very strong signal for β-sheet aggregation for VLITL at pH 2 (full line) and pH 8 (dotted line) at its C terminus. (B) PASTA2 predicted that VLITL is likely to stabilize the cross-β core of fibrillar aggregates and predicts parallel in-register intermolecular β-sheets for VLITL.
In silico studies of human Aα-chain amyloidogenic frameshift variants. (A) The TANGO aggregation scores of Phe521Leufs variant exhibited a very strong signal for β-sheet aggregation for VLITL at pH 2 (full line) and pH 8 (dotted line) at its C terminus. (B) PASTA2 predicted that VLITL is likely to stabilize the cross-β core of fibrillar aggregates and predicts parallel in-register intermolecular β-sheets for VLITL.
VLITL responsible for the amyloidogenic property of Phe521Leufs-derived peptides
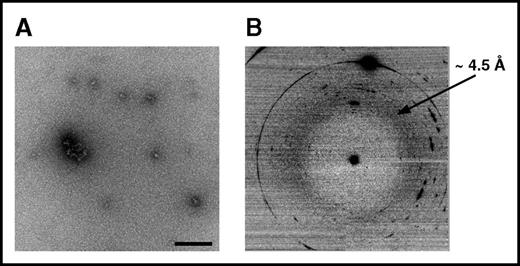
To experimentally verify that VLITL is the major amyloid sequence determinant of Aα-chain frameshifts, we generated synthetic Aα-chain-derived peptides containing the predicted amyloid-prone VLITL motif and compared their capacity for fibril formation with that of their VLITL-deleted counterparts. To this end, we designed AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG, the 49-mer full-length Phe521Leufs peptide identified in the AFib patient’s deposits, and its corresponding VLITL-deleted control (AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G). We also investigated the amyloid propensity of the ASSQIQRNPVLITLG peptide, common to all amyloidogenic Aα-chain variants, and its VLITL-deleted counterpart ASSQIQRNP_G. Fibrillogenesis experiments were performed under the same physiological conditions, and amyloid formation was evaluated using ThT, a dye binding to β-sheet aggregates with a characteristic maximum emission fluorescence intensity at 485 nm, TEM to determine the morphological features of aggregates, and XRD to ensure that aggregates possess the typical cross-β architecture.23,24 As shown in Figure 4A-D, AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG self-assembled into ThT-positive, β-sheet-enriched fibrillar aggregates exhibiting cross-β architecture that unambiguously defined amyloid fibrils. The ThT profile for AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G did not parallel that obtained for its corresponding amyloid VLITL counterpart. Despite a mild fluorescence intensity, maximum emission was observed at 510 nm instead of 485 nm, indicating that these aggregates have distinct dye-binding characteristics from typical amyloid fibrils (Figure 4A). Such a ThT profile has been associated with unstructured/amorphous aggregates.25 Consistent with ThT assay, TEM of AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G revealed spherical species that are distinguishable from the fibrillar morphology of aggregates formed by AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG (Figure 4B and Figure 5A). Even after an extended incubation period, AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G assembled into small spherical species without formation of fibrils (Figure 5A). In contrast, AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG organized into fibrils with a diameter of 11.4 ± 0.9 nm and twisted ribbonlike substructure (Figure 4B). XRD of AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG fibrils showed a typical “cross-β” molecular architecture with 4.72-Å sharp signal arising from the repeated β-strand spacing along the fibril axis and 11.5-Å signal corresponding to the stacking of β-sheets perpendicular to the fibril axis (Figure 4C-D). In contrast, the XRD pattern of AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G showed 1 diffuse reflection ring ∼4.5 Å arising from the mean distance of randomly distributed peptide chains (Figure 5B). Therefore, XRD provided definitive structural evidence that species formed by AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G lack ordered cross-β organization, the major diagnostic hallmark of amyloid. Experimental studies of ASSQIQRNPVLITLG demonstrated that this VLITL-containing peptide bound ThT with a characteristic amyloid maximum emission fluorescence intensity at 485 nm and formed fibrillar aggregates with a diameter of 13 ± 0.9 nm and cross-β molecular architecture (Figure 4E-G). In contrast, ASSQIQRNP_G did not exhibit any ThT spectral change (Figure 4E) and did not form aggregates (amorphous or fibrillar in nature) (data not shown), demonstrating that ASSQIQRNP_G does not form any kind of amyloid species under the same physiological conditions that the ASSQIQRNPVLITLG peptide does. Therefore, our experiments show that the 49-mer and 15-mer Phe521Leufs-derived peptides are readily amyloidogenic in vitro but lose their fibril-forming ability when VLITL is absent, supporting that their amyloidogenic behavior depends on VLITL amyloidogenic properties. Next, we experimentally verified whether VLITL itself forms amyloid in vitro. A ThT assay of VLITL showed typical amyloid enhancement of fluorescence emission at 485 nm (Figure 4I). In addition, TEM revealed twisted mature fibrils with a ribbonlike appearance (Figure 4J-K), and XRD confirmed that VLITL fibrils are amyloid with a highly ordered and well-defined cross-β structure (Figure 4L).
In vitro fibrillogenesis and structural analysis of Aα-chain frameshift-derived polypeptides identified in amyloid deposits in vivo. (A) ThT fluorescence assays performed on AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG (red curve) and AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G (blue curve). Characteristic enhanced ThT fluorescence at 485 nm was only observed for AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG, suggesting that this peptide formed amyloid β-sheet structures. In contrast, a spectral red shift at 510 nm was recorded for AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G, suggesting that it does not form typical amyloid β-sheet structures. (B) Transmission electron micrographs showed that AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG forms fibrillar aggregates, and AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G spherical aggregates. (C) Fibrils formed by AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG exhibited a meridional peak at 4.72 Å, indicating the spacing between β-strands within fibrils associated with an equatorial reflection at 11.50 Å. This microdiffraction pattern confirms the presence of the characteristic amyloid cross-β architecture constituted by intermolecular β-sheets with β-strands oriented perpendicular to the fibril axis. (D) An extracted radial profile from the two-dimensional pattern shown in panel C. The noisy background is because of the small angle used for the profile extraction to avoid the intense peaks from salts in the sample. (E) Data from ThT fluorescence assays performed on ASSQIQRNPVLITLG (red curve) and ASSQIQRNP_G (blue curve), revealing that only ASSQIQRNPVLITLG induces enhanced fluorescence at 485 nm and that only the peptide containing VLITL forms aggregates with β-sheet conformation. (F) Transmission electron micrographs showed that ASSQIQRNPVLITLG forms aggregates of fibrillar morphology. (G) XRD profiles of ASSQIQRNPVLITLG fibrils displaying the typical “cross-β” microdiffraction pattern of amyloid fibrils with a spacing of 4.75 Å along the meridional direction and a periodicity of 9.90 Å in the equatorial direction. (H) The in situ XRD pattern from a cut of the pathological kidney specimen of patient II.2 with in vivo amyloid fibrils, showing meridional (4.71 Å) and equatorial (10.0 Å) reflections. (I) A ThT fluorescence assay of VLITL with increased fluorescence intensity at 485 nm, demonstrating that the dye bound to β-sheet-enriched amyloid fibrils. (J and K) Transmission electron micrographs of mature VLITL fibrils with ribbonlike structures at different magnifications. Frayed ribbons are observed at the ends of the VLITL twisted fiber structures. The scale bar represents 200 nm. (L) The VLITL fibrils exhibited strong meridional (4.65 Å) and equatorial (10.7 Å) reflections characteristic of a cross-β pattern, confirming their amyloid nature.
In vitro fibrillogenesis and structural analysis of Aα-chain frameshift-derived polypeptides identified in amyloid deposits in vivo. (A) ThT fluorescence assays performed on AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG (red curve) and AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G (blue curve). Characteristic enhanced ThT fluorescence at 485 nm was only observed for AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG, suggesting that this peptide formed amyloid β-sheet structures. In contrast, a spectral red shift at 510 nm was recorded for AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G, suggesting that it does not form typical amyloid β-sheet structures. (B) Transmission electron micrographs showed that AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG forms fibrillar aggregates, and AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G spherical aggregates. (C) Fibrils formed by AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG exhibited a meridional peak at 4.72 Å, indicating the spacing between β-strands within fibrils associated with an equatorial reflection at 11.50 Å. This microdiffraction pattern confirms the presence of the characteristic amyloid cross-β architecture constituted by intermolecular β-sheets with β-strands oriented perpendicular to the fibril axis. (D) An extracted radial profile from the two-dimensional pattern shown in panel C. The noisy background is because of the small angle used for the profile extraction to avoid the intense peaks from salts in the sample. (E) Data from ThT fluorescence assays performed on ASSQIQRNPVLITLG (red curve) and ASSQIQRNP_G (blue curve), revealing that only ASSQIQRNPVLITLG induces enhanced fluorescence at 485 nm and that only the peptide containing VLITL forms aggregates with β-sheet conformation. (F) Transmission electron micrographs showed that ASSQIQRNPVLITLG forms aggregates of fibrillar morphology. (G) XRD profiles of ASSQIQRNPVLITLG fibrils displaying the typical “cross-β” microdiffraction pattern of amyloid fibrils with a spacing of 4.75 Å along the meridional direction and a periodicity of 9.90 Å in the equatorial direction. (H) The in situ XRD pattern from a cut of the pathological kidney specimen of patient II.2 with in vivo amyloid fibrils, showing meridional (4.71 Å) and equatorial (10.0 Å) reflections. (I) A ThT fluorescence assay of VLITL with increased fluorescence intensity at 485 nm, demonstrating that the dye bound to β-sheet-enriched amyloid fibrils. (J and K) Transmission electron micrographs of mature VLITL fibrils with ribbonlike structures at different magnifications. Frayed ribbons are observed at the ends of the VLITL twisted fiber structures. The scale bar represents 200 nm. (L) The VLITL fibrils exhibited strong meridional (4.65 Å) and equatorial (10.7 Å) reflections characteristic of a cross-β pattern, confirming their amyloid nature.
Structural characteristics of AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G assemblies. (A) The morphological features of aggregates. Note that these aggregates are spherical and do not form fibrillar structures, in contrast to amyloid fibrils formed by AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG (Figure 4B). (B) XRD did not reveal a cross-β microdiffraction pattern, demonstrating that the aggregates are not amyloid.
Structural characteristics of AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNP_G assemblies. (A) The morphological features of aggregates. Note that these aggregates are spherical and do not form fibrillar structures, in contrast to amyloid fibrils formed by AFFDTASTGKTFPGFFSPMLGELSVRLSLGAQNLASSQIQRNPVLITLG (Figure 4B). (B) XRD did not reveal a cross-β microdiffraction pattern, demonstrating that the aggregates are not amyloid.
Structural similarities between in vitro and ex vivo Phe521Leufs-derived fibrils
The structures of Phe521Leufs-derived fibrils generated in vitro were compared with those formed in AFib patient kidneys (individual II.2). To this end, we performed ex situ XRD studies of Phe521Leufs fibrils using kidney cuts obtained without denaturing extraction procedures to preserve the natural state of aggregates formed in vivo, offering the opportunity to gain structural information on fibrils in their natural cellular context (Figure 4H).18 XRD showed that the signal of the equatorial reflection from natural fibrils was very close to that of fibrils generated by ASSQIQRNPVLITLG, recapitulating the structural features of renal AFib fibrils in physiological conditions (Figure 4C,G-H,L).26 Therefore, this mutant 15-mer Aα-chain-derived sequence likely governs the detailed structure of Phe521Leufs fibrils formed in patient kidneys and might constitute a valuable “minimalist” in vitro amyloid model.
Discussion
Here, in addition to reporting a new “private” Aα-chain amyloidogenic frameshift variant, the aim of this study was to focus on the molecular mechanisms by which fibrinogen Aα-chain frameshift variants become amyloidogenic in humans.
Our results show that VLITL is a key amyloid-prone motif located at the C-terminally mutant end of all Aα-chain frameshift sequences, conferring amyloidogenic properties to Aα-chain frameshift variants. In this study, we first characterized which part of the Aα-chain was deposited in the Phe521Leufs patient’s tissues, and we demonstrated that a short 49-mer peptide deriving from the Phe521Leufs allele formed the renal fibrils. These clinico-anatomopathological findings overlap those obtained from our French AFib kindred carrying the Val522Alafs variant, which had previously documented that a 49-amino-acid peptide encoded by the Val522Alafs allele was similarly deposited as fibrils in kidneys.4 More recently, Yazaki et al also confirmed that the carboxyl terminal region of the amyloidogenic Ser523Argfs variant, containing VLITL, constituted the AFib patient’s renal deposits.13 Therefore, 3 ex vivo biochemical analyses of amyloid deposits from AFib patients carrying 3 distinct frameshift variants of Aα-chain concordantly established that only the C-terminal fragments of Aα-chain frameshift variants contribute to amyloid formation, and not the wild-type Aα-chain. We then tested the hypothesis that these highly similar Aα-chain C-terminal mutant sequences, specific to amyloidogenic variants, might contain segments with a high propensity to self-aggregate into β-sheets rendering mutant Aα-chains amyloidogenic. Our in silico analysis predicted that the C-terminal end of all mutant Aα-chains contained a major amyloid hot spot, VLITL. This prediction was in good concordance with the fact that VLITL was found within renal amyloid deposits of Phe521Leufs-, Val522Alafs-, and Ser523Argfs-AFib patients.4,13 To experimentally verify that VLITL is a major β-sheet signal of Aα-chain frameshift variants, we evaluated the ability of synthetic Phe521Leufs-derived peptides containing the VLITL motif to polymerize into β-sheets in vitro. We also compared their capacity for fibril formation with that of their VLITL-deleted counterparts. We provide in vitro evidence that VLITL is a fibril-forming motif, necessary for the β-sheet arrangement of the full-length Aα-chain peptide deposited as extracellular fibrils in the Phe521Leufs patient’s kidneys, whereas its absence abrogates fibril formation of Phe521Leufs-derived peptides. Therefore, combined in vitro and in vivo experiments support that the amyloidogenic behavior of the Phe521Leufs variant depends on the amyloidogenic properties of VLITL. The location of VLITL at the extreme end of renal fibrils probably renders this motif easily accessible for intermolecular interactions in vivo. VLITL satisfies 2 major criteria that are recognized as essential for triggering amyloid formation: high β-sheet propensity and appropriate position within the mutant Aα-chains for nucleating fibril formation.27,28 It is important to note that Phe521Leufs-derived peptides form amyloid fibrils in “physiological” experimental conditions. On this basis, it was therefore particularly relevant to compare the structural characteristics of Phe521Leufs-derived fibrils formed in vitro with those formed in the Phe521Leufs patient’s kidneys. To this end, XRD analysis of Aα-chain-fibrils from Phe521Leufs patients was performed “in situ”, directly on the pathological renal tissue without denaturating extraction procedures, to preserve the natural state of Aα-chain aggregates.18 This structural analysis revealed that ASSQIQRNPVLITLG fibrils closely reproduced the β-sheet structural organization of Aα-chain fibrils assembled in their natural cellular context. Therefore, ASSQIQRNPVLITLG, the mutant portion common to all amyloidogenic frameshifts, is a “minimalist” in vitro Aα-chain model suitable for all amyloidogenic Aα-chain frameshift variants. This in vitro Aα-chain model might be useful for testing antiamyloid agents targeting the VLITL motif because we showed that deleting VLITL disrupts fibril aggregation propensity of synthetic Phe521Leufs-derived peptides. Also, this in vitro model can be useful to conduct high-resolution structure investigations to shed light on the precise atomistic structure of Aα-chain frameshift-derived fibrils.
The reasons why fragments of the mutant Aα‐chain C terminus accumulate and form amyloid in kidneys, and why the mutant Aα‐chain could not be detected in the plasma of AFib patients, are not understood,4 but it has been proposed that it can be the consequence of an accelerated metabolism of the mutant Aα‐chain.4 In case of Aα‐chain dysfibrinogenic variants, experiments carried out on the His494fs and Ala499fs frameshift variants, each introducing in their mutant C-terminal part a novel unpaired cysteine residue, revealed that these mutant Aα‐chains circulated as disulfide-linked complexes with albumin. More importantly, it has been shown that these abnormal Aα-chain-albumin complexes directly altered the fibrin clot structure, conferring to the patients a dysfibrinogenic phenotype, clinically apparent as recurrent episodes of thromboembolism.21,22,29 Unlike Aα-chain dysfibrinogenic variants, the amino acid composition of the mutant C terminus of amyloidogenic frameshift variants does not contain a novel cysteine residue; thus, it is unlikely they are able to form abnormal disulfide conjugates with albumin. This could explain why, despite lacking Lys556, Lys580, and Lys601 in the αC-domain important for factor XIII cross-linking, AFib patients do not show clinical and biological evidence of clotting disorders. Further, we can speculate that these “albumin-free” amyloidogenic Aα‐chains might be more susceptible to undergo an aberrant proteolytic cleavage, yielding high concentrations of a catabolic intermediate containing the amyloid-prone VLITL motif serving as an amyloid core to initiate fibrillogenesis in the cellular environment of the kidney. Consistent with data from the literature, it is unlikely a coincidence that no mutations involving a cysteine residue in the Aα-chain have been associated with AFib, while they explain a large number of congenital dysfibrinogenemias associated with circulating fibrinogen-albumin conjugates (http://site.geht.org/base-fibrinogene/). An instructive example is given by fibrinogen Dusart, caused by the replacement of arginine 554 by cysteine (Arg554Cys) in the Aα-chain, which is associated with Aα-chains-554C-albumin complexes and recurrent episodes of thrombosis,30,31 while replacement of Arg554 by leucine (Arg554Leu) causes renal AFib amyloidosis.2 The 6 polypeptide chains of the normal fibrinogen molecule are covalently linked by numerous interchain disulfide bonds, with no free sulfydryl groups,1 supporting that an unpaired cysteine residue in the fibrinogen molecule might be critical on overall clot structure.
In summary, we provide compelling evidence that VLITL is part of Phe521Leufs fibrils formed in vivo and is a major fibril-forming motif necessary for β-sheet arrangement of the full-length Aα-chain Phe521Leufs peptide identified in the AFib patient’s kidneys. This VLITL amyloid motif, exclusively present at the C-terminal end of amyloidogenic Aα-chain frameshift sequences, and also previously identified in amyloid deposits of Val522Alafs- and Ser523Argfs-AFib patients support that VLITL is predictive of high risk of Aα-chain amyloid formation. This finding sheds light on the as yet unresolved issue of the mechanisms underlying frameshift Aα-chain amyloidogenesis, yielding an uncommon example among hereditary amyloidoses.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the family members for their participation in this study and l’Association Française contre l’Amylose. The authors thank Fabrice Senger and Marie-Paule Ramée for technical assistance with light microscopy and TEM, respectively; Céline Leroux for her assistance with FGA sequencing; and J. D. Theis for his assistance in proteomics analysis at the Mayo Clinic.
This work was supported in part by grants from l’Association Française contre l’Amylose.
Authorship
Contribution: P.L.P. collected the blood samples of the family, interpreted the clinical data, and supervised histology and immunohistochemistry analysis; C.G. and R.G. performed and analyzed the in vitro fibrillogenesis studies; F. Briki and J.D. performed and analyzed the XRD studies; N.R.-L. and L.M. performed and analyzed the microscopy and immunohistochemical analysis; A.D. performed the LMD/MS analysis; C.B. performed the molecular screening; F. Bridoux and M.D. contributed to the discussion of the data; G.G. contributed to the discussion, provided critical review of the manuscript, and edited and approved the final version of the manuscript; B.N. contributed to the preparation of the manuscript and prepared the figures; P.D. and S.V. analyzed in silico calculations and designed in vitro experiments; and S.V. supervised all aspects of this work, developed the whole idea, designed the experiments, collected and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sophie Valleix, Laboratoire de Génétique Moléculaire, Hôpital Necker-Enfants Malades, 75015 Paris, France; e-mail: sophie.valleix@aphp.fr.