Key Points
Histones promote in vitro erythrocyte aggregation, sedimentation, fragility, and spleen retention in a concentration-dependent manner.
Histones induce in vivo anemia, an increase in splenic hemoglobin content, as well as thrombocytopenia and leukopenia within a few minutes.
Abstract
Extracellular histones have been shown to play an important pathogenic role in many diseases, primarily through their cytotoxicity toward nucleated cells and their ability to promote platelet activation with resultant thrombosis and thrombocytopenia. In contrast, little is known about the effect of extracellular histones on erythrocyte function. We demonstrate in this study that histones promote erythrocyte aggregation, sedimentation, and using a novel in vitro shear stress model, we show that histones induce erythrocyte fragility and lysis in a concentration-dependent manner. Furthermore, histones impair erythrocyte deformability based on reduced passage of erythrocytes through an artificial spleen. These in vitro results were mirrored in vivo with the injection of histones inducing anemia within minutes of administration, with a concomitant increase in splenic hemoglobin content. Thrombocytopenia and leukopenia were also observed. These findings suggest that histones binding to erythrocytes may contribute to the elevated erythrocyte sedimentation rates observed in inflammatory conditions. Furthermore, histone-induced increases in red blood cell lysis and splenic clearance may be a significant factor in the unexplained anemias seen in critically ill patients.
Introduction
Histones are highly conserved, cationic proteins that play an integral role in DNA condensation and gene expression.1 However, multiple studies have indicated that extracellular histones and neutrophil extracellular traps (NETs), the predominant source of extracellular histones, play an important role in many pathologies, namely, sepsis,2 trauma,3 atherosclerosis,4 cardiac ischemia reperfusion injury,5 renal6 and liver injury,7 and autoimmune disorders.8 It is thought that extracellular histones mediate these pathological effects by being cytotoxic for nucleated cells, such as endothelial and epithelial cells,9 and by promoting platelet activation with resultant thrombosis and acute thrombocytopenia.10
In contrast to nucleated cells and platelets, there is limited literature describing the interaction between histones and erythrocytes. An early investigation reported that polycations, such as histones, agglutinate erythrocytes,11 and subsequently it was demonstrated that erythrocytes express phosphatidylserine on their surface after exposure to histones and, in doing so, become procoagulant.12 The focus of this study, therefore, was to investigate whether histones bind to erythrocytes and alter their biology and function in vitro and in vivo.
Study design
Human erythrocytes
Washed human erythrocytes from healthy donors were resuspended as a 20% (volume-to-volume ratio) suspension in phosphate-buffered saline (PBS)/0.1% bovine serum albumin before exposure to concentrations of calf thymus histones as indicated (Sigma-Aldrich, Sydney, NSW, Australia).
In vitro shear stress and splenic filtration assays
An automated 96-well microplate pipetting system (epMotion 5070; Eppendorf AG, Hamburg, Germany), using TS 50 tips with an aperture radius of 0.25 mm and MicroAmp Optical 96-Well Reaction Plates (Applied Biosystems, Norwalk, CT), was used to mimic the mechanical sheer stress encountered by circulating erythrocytes. Shear exposure was determined by the number of passages (5-40×) and the speed of passage (25-100 mm/s) through the TS 50 tips. The erythrocyte samples were incubated with the histones for 60 minutes at 37°C in 60% tonicity PBS/0.1% bovine serum albumin before shear stress exposure, and erythrocyte fragility was measured by hemoglobin release (optical density, 540 nm) after lysis. In parallel, an artificial human spleen assay was used in vitro to detect, by filtration, the deformability of erythrocytes13 after histone exposure.
Mice
The Australian National University Animal Experimentation Ethics Committee approved all animal experimental procedures; 5- to 8-week-old female BALB/c mice, bred at the Australian National University, were used in this study.
Analysis of histone-mediated effects in vivo
Mice were injected IV with histones (50 mg/kg) in PBS. Retro-orbital bleeds were performed, spleens were harvested at 5, 10, 20, and 30 minutes postinjection, and single-cell suspensions of splenocytes were prepared.14 Hematologic analysis of blood samples was subsequently performed with an ADVIA 2120i hematology analyzer (Siemens, Munich, Germany). The splenocyte hemoglobin content was quantified with a hemoglobin assay kit (Sigma-Aldrich).
Flow cytometry, ESR, and SEM
Histone-induced aggregation of erythrocytes was quantified by flow cytometry based on increases in forward scatter (FSC) and side scatter (SSC) or by increases in erythrocyte autofluorescence (FL-1 channel) using a BD FACSCalibur instrument (BD Biosciences, North Ryde, NSW, Australia). Aggregation of erythrocytes was also monitored by scanning electron microscopy (SEM)15 and by the erythrocyte sedimentation rate (ESR).16
Results and discussion
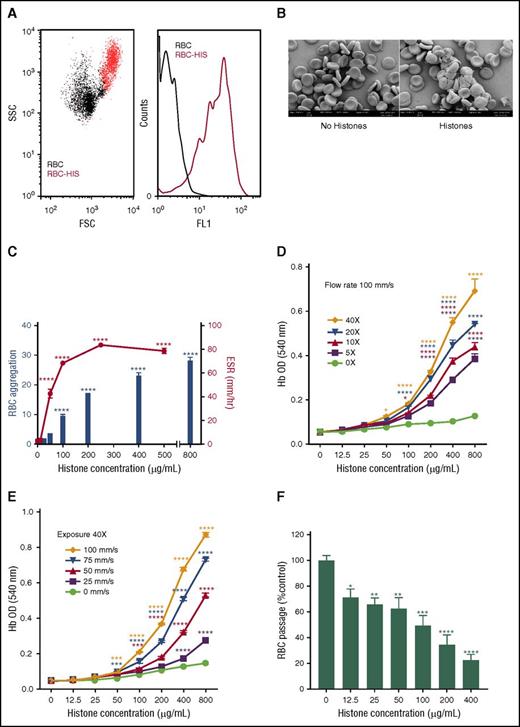
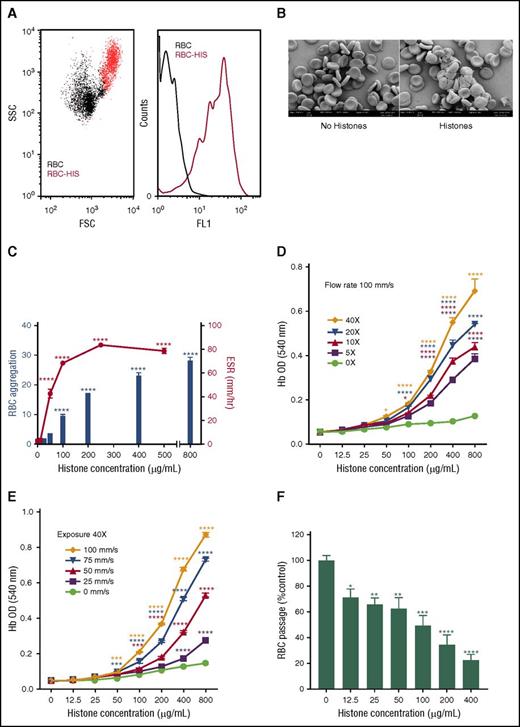
The initial studies revealed that when human erythrocytes were incubated with histones, substantial erythrocyte aggregation occurred that could be readily detected by flow cytometry based on increases in FSC and SSC and erythrocyte autofluorescence (Figure 1A), a result that was confirmed by SEM (Figure 1B). Subsequent experiments used erythrocyte autofluorescence and ESR to demonstrate that aggregation and increased sedimentation of erythrocytes induced by histones was concentration dependent and reached statistical significance at histone concentrations of ≥100 μg/mL and ≥50 μg/mL, respectively (P < .0001) (Figure 1C).
Extracellular histones mediate erythrocyte aggregation and induce erythrocyte fragility. (A) Flow cytometry detection of histone (HIS, 400 µg/mL)-induced erythrocyte (RBC) aggregation based on FSC and SSC plots (left: RBC alone, black dots, RBC-HIS, red dots) and erythrocyte autofluorescence histograms (right: FL-1 channel); autofluorescence of RBC alone is indicated by the black line and RBC-HIS by the red line. (B) SEM images of untreated (left) and histone-treated (right) human erythrocytes confirm that histones mediate erythrocyte aggregation. (C) Concentration dependence of histone-induced RBC aggregation and increased ESR, with RBC aggregation calculated as the fold increase in RBC autofluorescence relative to RBC alone. (D) Lysis of RBCs in the presence of different histone concentrations when exposed to 5 to 40 (5×, 10×, 20×, and 40×) passages through an epMotion 5070 automated 96-well microplate pipetting system at a constant flow rate of 100 mm/s or (E) a variable flow rate (25, 50, 75, and 100 mm/s) at a constant passage number (40×). Note that in the absence of shear, negligible lysis of erythrocytes occurred at all histone concentrations. (F) Erythrocyte retention in an artificial spleen in the presence of increasing histone concentrations. Data are expressed as the mean ± standard error of the mean (n = 3) and are representative of ≥3 separate experiments. *P ≤ .05; **P < .01; ***P < .001; ****P < .0001 (1-way analysis of variance with Tukey’s multiple comparisons test).
Extracellular histones mediate erythrocyte aggregation and induce erythrocyte fragility. (A) Flow cytometry detection of histone (HIS, 400 µg/mL)-induced erythrocyte (RBC) aggregation based on FSC and SSC plots (left: RBC alone, black dots, RBC-HIS, red dots) and erythrocyte autofluorescence histograms (right: FL-1 channel); autofluorescence of RBC alone is indicated by the black line and RBC-HIS by the red line. (B) SEM images of untreated (left) and histone-treated (right) human erythrocytes confirm that histones mediate erythrocyte aggregation. (C) Concentration dependence of histone-induced RBC aggregation and increased ESR, with RBC aggregation calculated as the fold increase in RBC autofluorescence relative to RBC alone. (D) Lysis of RBCs in the presence of different histone concentrations when exposed to 5 to 40 (5×, 10×, 20×, and 40×) passages through an epMotion 5070 automated 96-well microplate pipetting system at a constant flow rate of 100 mm/s or (E) a variable flow rate (25, 50, 75, and 100 mm/s) at a constant passage number (40×). Note that in the absence of shear, negligible lysis of erythrocytes occurred at all histone concentrations. (F) Erythrocyte retention in an artificial spleen in the presence of increasing histone concentrations. Data are expressed as the mean ± standard error of the mean (n = 3) and are representative of ≥3 separate experiments. *P ≤ .05; **P < .01; ***P < .001; ****P < .0001 (1-way analysis of variance with Tukey’s multiple comparisons test).
To further explore the consequences of histone binding to erythrocytes, a novel shear flow model was developed wherein erythrocytes incubated with histones in a 60% hypotonic solution were subjected to increasing exposure (passage number) (Figure 1D) or flow rates (Figure 1E), and the extent of lysis was then determined by measuring hemoglobin in the supernatant. Similar experiments performed under isotonic conditions gave comparable but less marked fragility results (data not shown). These experiments showed that histones increase erythrocyte fragility in a concentration-dependent manner, with both the exposure level and flow rate significantly affecting the extent of histone-mediated lysis (Figure 1D-E). The experimental flow conditions used in this assay were within the normal physiological range, because the shear rates, which involved a 0.25-mm radius tube, were similar to those encountered in the vasculature,17 and histone levels in trauma patients have been reported to be as high as 250 μg/mL.18,19 Furthermore, histone-induced fragility is not restricted to erythrocytes; a histone level of 400 µg/mL causes an ∼50% loss of leukocytes under shear (data not shown).
Erythrocytes are structured for flexibility that enables them to deform and pass through small capillaries, and thus we investigated whether histone binding to erythrocytes would compromise their deformability. Using an artificial spleen model previously used in malaria-infected erythrocyte deformability studies,13 we examined erythrocyte retention within the artificial spleen after exposure to increasing concentrations of histones (Figure 1F). The number of erythrocytes that were able to pass through the artificial spleen significantly diminished as the histone concentration increased. This change was not due to erythrocyte lysis, because this model does not apply a significant level of shear force and, consequently, the free hemoglobin content of eluates did not increase when histones were present (data not shown). Furthermore, erythrocytes exposed to histone concentrations insufficient for induction of aggregation (ie, 12.5 and 25 μg/mL) were still retained by the artificial spleen, implying that retention is not merely due to aggregation, but involves additional factors, such as erythrocyte deformability.
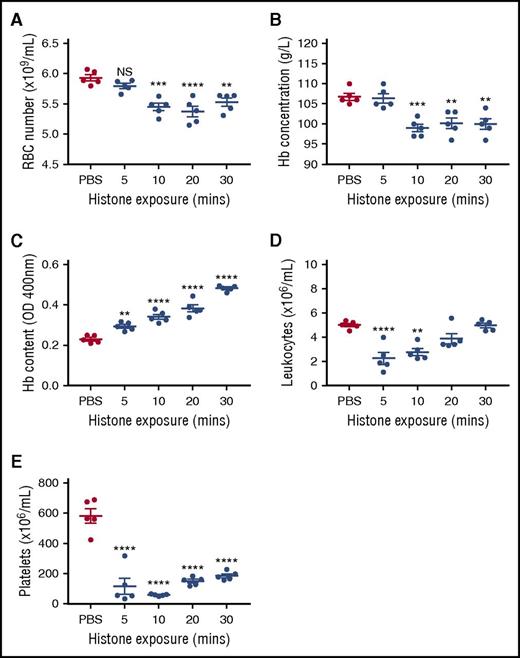
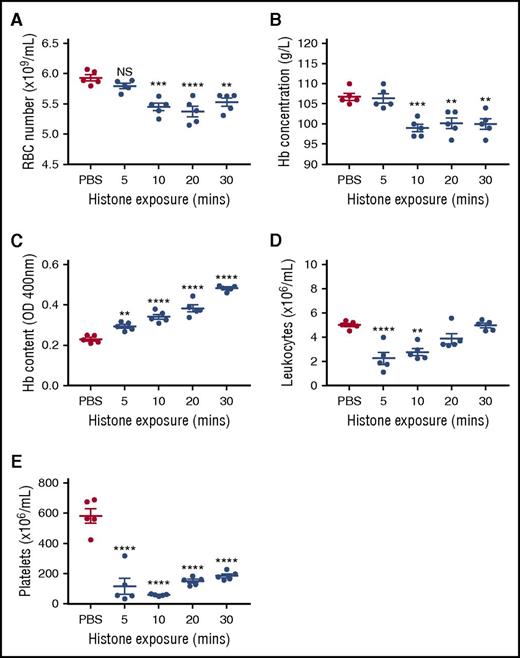
Because in vitro conditions cannot fully replicate the complexities inherent in vivo, we injected mice IV with 50 mg/kg of histones to determine the impact on erythrocytes 5 to 30 minutes post–histone injection. Circulating erythrocyte numbers and hemoglobin content were significantly reduced at 10, 20, and 30 minutes post–histone injection (Figure 2A-B). Additionally, profound leukopenia and thrombocytopenia also occurred in these mice (Figure 2D-E). Histone-induced thrombocytopenia has been reported,10 however, histone-induced leukopenia has not previously been identified. Consistent with splenic removal of damaged erythrocytes, splenic erythrocyte content, based on hemoglobin levels, was seen to significantly increase at all time points studied, concomitant with decreasing circulating erythrocyte numbers (Figure 2C).
Histones induce transient anemia and enhance splenic uptake of erythrocytes. Five groups of BALB/c mice were injected IV with 50 mg/kg of histones or PBS, and 1 group was euthanized at each 5, 10, 20, and 30 minutes postinjection for determination of (A) the number of circulating erythrocytes, (B) plasma hemoglobin (Hb) concentration, (C) Hb uptake by the spleen, (D) circulating leukocyte numbers, and (E) circulating platelet numbers. In this experiment, 5-week-old mice were used, but very similar results were obtained with 8-week-old animals (data not shown). Results are representative of 3 separate experiments. Data are expressed as the mean ± standard error of the mean (n = 5). **P < .01; ***P < .001; ****P < .0001 (1-way analysis of variance with Tukey’s multiple comparisons test). NS, not significant.
Histones induce transient anemia and enhance splenic uptake of erythrocytes. Five groups of BALB/c mice were injected IV with 50 mg/kg of histones or PBS, and 1 group was euthanized at each 5, 10, 20, and 30 minutes postinjection for determination of (A) the number of circulating erythrocytes, (B) plasma hemoglobin (Hb) concentration, (C) Hb uptake by the spleen, (D) circulating leukocyte numbers, and (E) circulating platelet numbers. In this experiment, 5-week-old mice were used, but very similar results were obtained with 8-week-old animals (data not shown). Results are representative of 3 separate experiments. Data are expressed as the mean ± standard error of the mean (n = 5). **P < .01; ***P < .001; ****P < .0001 (1-way analysis of variance with Tukey’s multiple comparisons test). NS, not significant.
Collectively, these data provide compelling evidence that histones cause significant erythrocyte damage by inducing erythrocyte aggregation, increasing erythrocyte lysis under shear stress, and reducing erythrocyte deformability. In vivo, these processes resulted in anemia and accelerated erythrocyte removal by the spleen. Histones present in NETs may also contribute to these processes, because erythrocytes can associate with NETs.20 Furthermore, lysis of erythrocytes with the release of heme may further exacerbate the situation, because heme has been demonstrated to induce NETosis.21 Nevertheless, the murine erythropoietic system rapidly replenished erythrocytes after a single bolus injection of histones, with levels returning to the normal range within 1 hour (data not shown). In the acutely ill patient, however, when comorbidities are present, when the production of histones may be continuous over a period of days, or when histone-binding plasma proteins, such as albumin, become depleted,22 histone-associated anemia is more likely to develop and require correction. The results presented in this study, therefore, provide evidence for several mechanisms of histone-induced erythrocyte loss in histone-associated pathologies that may help to explain the anemia observed with acute illness in the absence of obvious causes.23
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anna Bezos and Anna Browne for help with laboratory work and blood donation. The authors also thank Ross Stephens for reviewing the manuscript.
This work was supported in part by Sirtex Technology, Pty., Ltd.
Authorship
Contribution: F.K., C.H.O., and L.A.C. performed the experiments, with F.K. and C.H.O. making the greatest contribution; C.H.O., F.K., L.A.C., and C.R.P. wrote the paper; and all authors developed the experimental techniques and revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: C.R.P. received research funding from Sirtex Technology Pty., Ltd., for this project and holds stocks in the start-up companies Lipotek and Beta Therapeutics and in the publicly traded companies Biotron and TBG Diagnostics. The remaining authors declare no competing financial interests.
The current affiliation for P.M.L. is Laboratory of Malaria Immunology, Immunology Frontier Research Center, Osaka University, Osaka, Japan.
Correspondence: Christopher R. Parish, Cancer and Vascular Biology Group, Australian Cancer Research Foundation Department of Cancer Biology and Therapeutics, John Curtin School of Medical Research, Australian National University, Canberra, ACT 2601, Australia; e-mail: christopher.parish@anu.edu.au.
References
Author notes
F.K. and C.H.O. contributed equally to this study.