Abstract
BACKGROUND: Patients with end-stage renal disease (ESRD) on hemodialysis are at high risk of both thrombotic and bleeding events. Experimental and clinical data indicate that reducing factor XI (FXI), a key component of the intrinsic pathway, prevents thrombosis without causing bleeding. FXI activity can be lowered with IONIS-FXIRx, a 2nd generation antisense oligonucleotide that specifically reduces human FXI mRNA expression in the liver. This study sought to determine the safety, pharmacokinetics (PK) and pharmacodynamics (PD) of IONIS-FXIRx in participants with ESRD.
METHODS: In this Phase 2 multicenter study, we enrolled a total of 49 ESRD patients: (A) 6 participants receiving chronic in-center hemodialysis (HD) to receive an open-label single-dose of 300 mg IONIS-FXIRx either 5 minutes before or 10 minutes after HD and assessed the effects of HD on peak (Cmax) and the extent of exposure (AUC0-24hr) parameters of IONIS-FXIRx and (B) another 43 participants in a double-blind design to one of two multiple-dose regimens (200 mg or 300 mg) IONIS-FXIRx or placebo for 12 weeks with 12 weeks of additional follow up. In the double-blind portion, IONIS-FXIRx was administered subcutaneously ~10 minutes after HD. For PK, plasma trough concentration and apparent elimination half-life (t1/2λz) were evaluated. The PD parameters including FXI activity and antigen, aPTT, prothrombin time (PT), and INR were evaluated in the per-protocol population (N= 34) that was defined as all participants who received the complete protocol-specified administration of IONIS-FXIRx or placebo through week 8 and who did not have any major protocol violations. The rate and frequency of clotting on the HD filters and circuit were qualitatively measured with a clotting scale as an exploratory PD analysis.
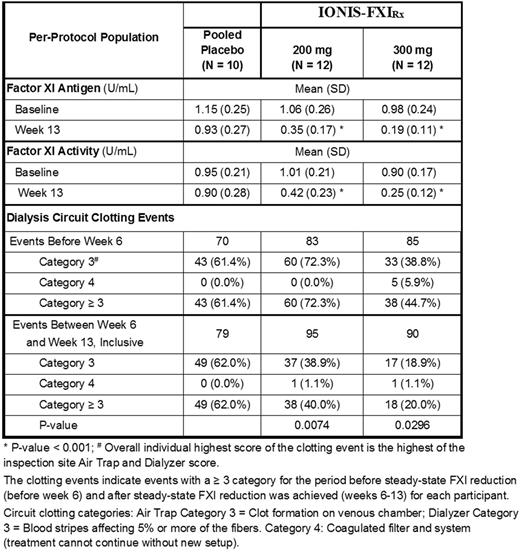
RESULTS: The PK cohort included 6 males (5 white) with a mean age of 61 years. The plasma PK profile of IONIS-FXIRx was not significantly altered whether injected before or after HD. After multiple dosing, plasma Cmax values were dose-dependent and similar between Day 1 (first dose) and Day 78 (last dose), suggesting no accumulation of IONIS-FXIRx in ESRD participants after 12-weeks of dosing. After treatment cessation, IONIS-FXIRx had an apparent elimination half-life of approximately 2 weeks. In the double-blind portion of the study, 22 (51.2%) of the participants were male, 24 (55.8%) were white and the mean age was 59 years. Attenuation of FXI activity was associated with a reduction in the incidence of severe clotting events on both the air filter and dialyzer (Table). The reduction in severe clotting events was observed when FXI activity was < 0.4 U/mL. Consistent with prior observations in healthy subjects, IONIS-FXIRx prolonged aPTT in a time and dose-dependent manner with maximum mean percent changes from baseline of 53.3% (p < 0.05) for the 200 mg dose group and 75.6% (p < 0.05) for the 300 mg dose group at Week 14, but had no effect on PT. Treatment emergent adverse events (TEAEs) were mostly mild; participants reporting serious TEAEs were 1 (16.7%) for the PK cohort, 4 (30.8%) for placebo and 3 (20.0%) each for the 200 mg and 300 mg IONIS-FXIRx dose groups. There were no drug-related SAEs and no drug-related major or clinically-relevant non-major bleeding events. Minor bleeds were primarily observed in the 300 mg multiple-dose group at AV fistula/graft sites but did not correlate with reduction of FXI activity. No clinically significant reductions in platelet counts were reported during the study.
CONCLUSIONS: This study demonstrated that (i) HD had no effect on IONIS-FXIRx PK, (ii) IONIS-FXIRx was well tolerated and produced sustained, and dose-dependent reductions in FXI antigen and activity, and (iii) IONIS-FXIRx reduced severe dialysis circuit clotting events beyond standard heparin use. Collectively, these data support further evaluation of IONIS-FXIRx as a potentially safe, effective antithrombotic therapy in ESRD patients on HD.
Bethune: Ionis Pharmaceuticals: Employment. Walsh: Population Health Research Institute: Employment; Ionis Pharmaceuticals: Consultancy. Jung: Ionis Pharmaceuticals: Employment. Yu: Ionis Pharmaceuticals: Employment. Geary: Ionis Pharmaceuticals: Employment. Bhanot: Ionis Pharmaceuticals: Employment.
Author notes
Asterisk with author names denotes non-ASH members.