Abstract
Introduction
Platelet (PLT) transfusion is an important treatment for acutely bleeding patients. PLTs contribute to hemostasis not only by forming a primary hemostatic plug, but also by their unique ability to apply contractile forces on fibrin, thereby reducing the blood flow at the wound site. Clot retraction is a poorly defined phenomenon, and the effect of storage conditions on clot retraction is understudied. We measured clot retraction using an unconfined retraction model employed in a clinical setting, and investigated the effects of PLT storage conditions on clot structure and composition using immunohistochemistry of retracted clots and serum analysis.
Methods
Apheresis PLT (AP) concentrates were collected from 4 healthy donors and stored for 5 days at RT (22-24°C) with agitation or stationary at 4C (4°C). Cold PLT samples were warmed for 30 mins to RT before performing experiments and PLTs were isolated at 800xg for 10 mins with 1μM Prostaglandin I2 (PGI2). Fresh Frozen Plasma (FFP) was used to eliminate plasma degradation effects during storage. Clotting was initiated with 20mM Ca2+ in 1 mL PRP in glass test tubes and incubated at 37°C. After 2 h, the clots were weighed and preserved by embedding in OCT (Optimum Cutting Temperature compound) at -80C. These clots were further sectioned into 5 um sections and stained for fibrinogen and PLT CD61. The sections were imaged using confocal microscopy and Transmission Electron Microscopy to study the structure and PLT-fibrin interaction in retracted clots. The exuded serum after retraction was used to measure PLT concentrations and D-Dimer levels to understand the relative incorporation of PLTs and contribution of fibrinolysis to clot structure. PLTs adhesion assays were used to investigate PLT spreading and adhesion capabilities to support active fibrin interaction. Mechanical properties of these clots were measured using tensile testing, and Atomic Force Microscopy was used to quantify the force generation in these clots.
Results
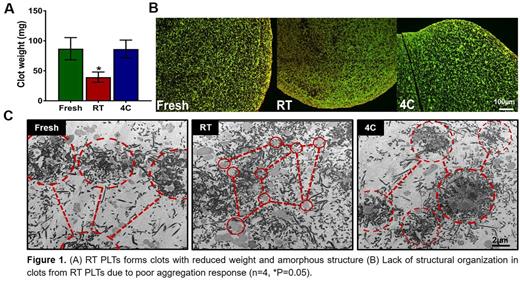
Clot retraction studies indicate that 4C-stored PLTs have clot retraction properties similar to fresh PLTs. The retracted clots from 4C-stored PLTs form heavier clots with PLTs forming nucleation centers with a highly organized structure. However, RT-stored PLTs form significantly smaller clots with a disorganized, amorphous structure (Fig 1A). The reduced weight of the clots formed by RT PLTs appears to be related to poor adhesion, spreading and aggregation responses (Fig 1B).
Conclusion
Our data suggest that PLTs adhesion, spreading and aggregation responses are critical to supporting active interactions with fibrin to form organized clots. We have shown that 4C-stored PLTs form stable clots similar to those formed by fresh PLTs with structural attributes of improved retraction compared to RT PLTs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.