Abstract
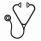
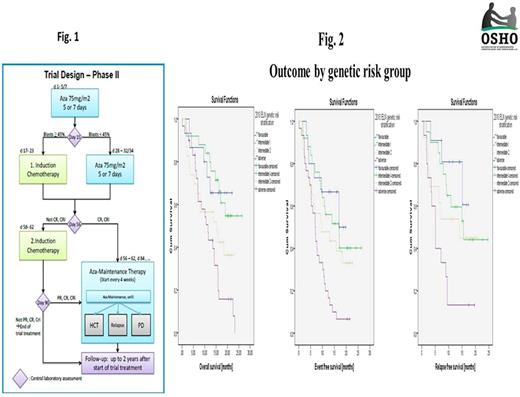
Age >60 years (y) is independently associated with poorer outcomes in AML. An increasing incidence of s-AML and t-AML as well as age-related genetic abnormalities increase the likelihood of resistance to treatment. After intensive chemotherapy (IC), complete remission (CR/CRi) rates of 48-51% and a day (d) 90 mortality of 19-27% are reported (Niederwieser et al, ASH 2016). In pts >65y, azacitidine (AZA), a hypomethylating agenet, yields CR/CRi rates of 27.8% and a one-year survival (OS) of 46.5% (Dombret et al, Blood 2015). In the RAS-AZIC (DRKS00004519) study, a multicenter, investigator-initiated, combined phase I/II trial, AZA was integrated with IC in an individualized response-based approach in pts >60y with newly diagnosed AML. The rationale was a treatment sequence with a low probability of toxicity and a high probability of efficacy. Outcomes are presented. Patients and methods: In the phase I part, upfront AZA (75 mg/m2/day s.c) for 7 d followed by IC (Mitoxantrone 10 mg/m2/day d 1-3 and cytarabine 1g/m2/BID d1,3,5,7) on d17 was declared admissible through a 3+3 design (n=9). Between June, 2014 and May, 2016, 103 pts received priming with AZA followed by IC or AZA in phase II (Fig. 1) based on bone marrow (BM) blasts d15 (<45 vs >45%) and CR/CRi d56, previousely identified as predictors for long-term response to AZA in AML (Al-Ali et al, Leuk Lymph 2012). AZA maintenance was offered to pts in remission until relapse or allogeneic HCT. Primary objective was d90 response (OR) [CR/CRi/PR] in the 109 pts who received the maximal tolerated priming dose of AZA. Safety, d90 mortality, OS, EFS; RFS were further end points. Protocol treatment was considered non-inferior to standard IC if, on an intention-to-treat basis, an OR >61% was reached. Adverse events (AEs) were reported according to the NCI CTCAE 4.03. All pts gave written informed consent. Results: Median age was 70 (61-85) y. A median of 6 comorbidities/patient were reported [hypertension, n=55 (50%); cardiac disorders, n=47 (43%); diabetes mellitus, n=26 (24%); history of non-myeloid neoplasia, n=22 (20%)]. Median baseline BM blasts and WBC were 50% and 4.3 (range 0.4-153)x109/L respectively. Type of AML was de novo AML (63.3%), s-AML (23.9%), and t-AML (12.8%). Genetic risk according to the 2010 ELN stratification was high in 28%, int-I and II each in 23.4%, favorable in 14%; and not evaluable in 11.2%. All pts received first-line AZA, 90% and 78% of pts received protocol assigned treatment based on d15 BM blasts and d56 response respectively [AZA1+AZA2, n=24 (22%); AZA1+AZA2+IC1, n=30 (27.5); AZA1+ IC1, n=35 (32.2%); AZA1+ IC1+IC2, n=9 (8.3%)]. Hematologic and non-hematologic AEs lead to a postponement of response-adapted IC1 in 5.4% and 8% of pts respectively. Overall, infections were the most frequent non-hematologic AE [> grade 2 in 26.5%, 21%, and 53.5% of pts under AZA1, AZA2, and IC respectively]. Day 90 OR and mortality were 63.3% [CR/CRi 56.9%; PR 6.4%] and 10% respectively. OR was 73%, 80%, 52%, and 50% in favorable, int-I, int-II, and high-risk genetics respectively (p=0.07). One-year OS, EFS, and RFS were 63.8% (95% CI, 55% to 73%), 46% (95% CI, 36% to 55%), and 50% (95% CI, 38% to 62%) respectively. Age, baseline BM blasts, WBC, type of AML, FLT3/NPM1 mutational status,and d15 blasts <45%/>45% had no impact on OR and OS. In pts >75y, OS was 69% compared to 59.3% in pts < 64y (p=0.4). OS and EFS were associated with d90 OR. After a median follow-up of 19.7 months (m), median survival and EFS times for responders were 23.2 and 15.8 vs. 6.7 and 4.7m for non-responders respectively (p<000). High risk genetics were associated with a poorer OS [median survival times were not reached for favorable and int-I; 15.3 and 11.4m in int-II and high risk respectively] (p=0.002). Similar associations were found for EFS (p=0.002) and RFS (p=0.005) (Fig. 2). Conclusion: With a response-adapted approach in elderly patients with AML, favorable outcomes with low toxicity are possible even in patients >70 years. The well-known negative impact of pretreatment variables such as increasing age or type of AML on outcome could, at least partly, be ameliorated with treatment. The results of this trial emphasize that age alone should not be the determinant of treatment decisions.
Jaekel: Celgene: Other: Travel support. Maschmeyer: Boehringer-Ingelheim: Honoraria; AstraZeneca: Honoraria; Astellas: Honoraria; Janssen-Cilag: Honoraria; Basilea: Honoraria; Bristol-Meyers Squibb: Honoraria; Celgene: Honoraria; Merck-Serono: Honoraria; Pfizer: Honoraria, Travel support; Gilead: Honoraria; Amgen: Honoraria; Bristol-Meyers Squibb: Other: Travel support. Al-Ali: Gilead: Consultancy, Honoraria; Alexion: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract