Abstract
Background
Peripheral T cell lymphomas (PTCL) are a heterogeneous group of lymphomas that have poor outcomes with current frontline chemotherapeutic strategies. Cyclophosphamide, vincristine, doxorubicin and prednisone, with or without the inclusion of etoposide (CHO(E)P), remains the standard of care, although scarce data exist regarding the benefit of intensive regimens, including hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternating with high dose methotrexate/cytarabine (HyperCVAD). This multicenter study aims to characterize presenting features, prognostic factors and the influence of frontline therapy in patients (pts) with PTCL.
Methods
We retrospectively analysed presenting features, frontline therapy and outcomes of all pts diagnosed with PTCL at the 3 tertiary referral centers within Western Australia from 2005-2014. Overall survival (OS) and progression free survival (PFS) were calculated by the Kaplan Meier method. Univariate comparisons were made by log rank test and multivariate analysis (MVA) performed using step-wise Cox regression. Comparisons between categorical variables were made using Fisher's exact tests.
Results
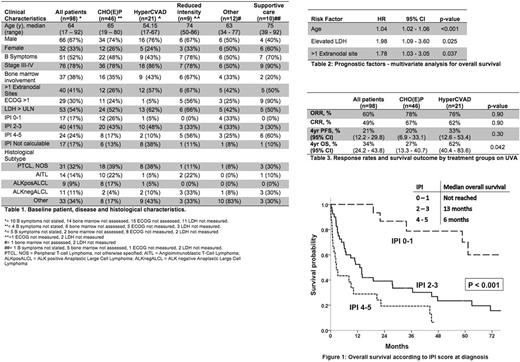
A total of 98 pts were included in the analysis, with a median follow-up of 4.6 years (range 0.1 - 8.6). Median age was 64 years and 78% presented with advanced stage (III-IV) disease. Complete pt characteristics and histological subtypes are presented in Table 1.
Most pts were treated with CHO(E)P (47%) or HyperCVAD (21%), with a smaller proportion receiving reduced intensity chemotherapy (9%), other therapy (12%) or supportive care alone (10%).
Four year PFS was 21% (95% CI 12.2 - 29.8%) and 4 year OS was 34% (24.2 - 43.8%). Excluding pts treated with palliative intent, the overall response rate (ORR) was 73%, with a complete response rate (CRR) of 59%.
OS was strongly predicted by International Prognostic Index score (IPI) (Figure 1). In MVA, age, elevated LDH and >1 extranodal sites were found to be significantly associated with survival, whereas marrow involvement, B symptoms and stage were not (Table 2).
We then restricted the analysis to pts treated initially with either CHO(E)P or HyperCVAD to compare outcomes between these two commonly used regimens at our centers. Response rates were similar with an ORR/CRR to CHO(E)P of 78%/67%, and 76%/61% to HyperCVAD (p=0.90). Likewise, 4yr PFS did not differ significantly between the two regimens - CHO(E)P: 20% (6.9 - 33.1%) vs. HyperCVAD: 33% (12.6 - 53.4%), p = 0.30. On univariate analysis (UVA), 4yr OS was 27% (13.3 - 40.7%) for CHO(E)P and 62% (40.4 - 83.6%) for HyperCVAD, p = 0.042. On MVA adjusted for age, LDH and extranodal sites, there was no significant difference between treatment groups (Table 3).
Consolidation stem cell transplantation (SCT) following response to first line chemotherapy was performed in only 6 (10%) pts and occurred more frequently in pts initially treated with HyperCVAD (4/16 [25%] vs. 2/36 [6%], p=0.074). In relapsed or refractory disease, pts treated initially with HyperCVAD were more likely than those treated with CHOP to receive salvage chemotherapy (11/12 [92%] vs. 20/33 [61%], p=0.046) and have SCT incorporated into subsequent lines of therapy (7/12 [58%] vs. 4/33 [12%], p=0.003).
Pts treated with reduced intensity chemotherapy had poor outcomes, with median OS of 3 months. Those receiving only supportive care had a median OS of 0.8 months.
Conclusion
Whilst pts with PTCL presenting with low risk (IPI 0-1) disease have favourable outcomes, the majority present with higher risk disease and have poor long-term survival with current frontline therapy. Survival in our cohort was strongly associated with IPI risk factors including age, elevated LDH and multiple extranodal sites.
The use of HyperCVAD did not improve response rate or PFS when compared to CHO(E)P in our pts. The superior OS noted on UVA for this group was lost on MVA, and appears to be explained by selection of younger, fitter pts, more of whom underwent salvage therapy, including autologous or allogeneic SCT, at time of progression. Whilst some expert guidelines support SCT as part of initial therapy (Moskowitz, Blood 2014), this was seldom performed at our centers.
Our results do not support the frontline use of HyperCVAD over CHO(E)P in PTCL. Given poor outcomes with current chemotherapeutic options, alternative induction regimens incorporating novel agents are urgently required.
Cull: Amgen Australia: Other: travel expenses Lugano lymphoma conference 2017; Takeda Australia: Other: travel expenses Highlights of ASH march 2017, Brisbane.
Author notes
Asterisk with author names denotes non-ASH members.