Abstract
Introduction
Post-transplant lymphoproliferative disease (PTLD) arises in the setting of immunosuppression following solid organ transplantation (SOT) or hematopoietic stem cell transplantation. PTLD incidence ranges from 1 to 20% in SOT; mortality estimates of >30% make this entity a significant life-threatening complication of transplantation. PTLD is broadly categorized into four groups: early; polymorphic; monomorphic, including T/natural killer cell neoplasms and most B cell neoplasms; and classical Hodgkin lymphoma. The heterogeneity of PTLD histology, compounded by relative disease rarity and absence of a standard treatment approach, has made evaluating clinical outcomes in specific patient populations difficult. We analyzed data from a systematic review of the literature to investigate the impact of PTLD histologic subtype on survival in a large dataset.
Methods
Case series were identified on PubMed using the search terms "post-transplant lymphoproliferative disorder/disease," "PTLD", and "solid organ transplantation," with additional publications identified through reference lists of these papers. Inclusion criteria were: 1) articles published in English between January 1, 1974 and July 1, 2016 with pathology diagnosis and survival details; 2) adult cases following SOT. Patient characteristics, immunosuppressive regimen, treatment, survival, and follow-up time of 306 cases were extracted from 94 articles and combined with 11 cases from our institution.
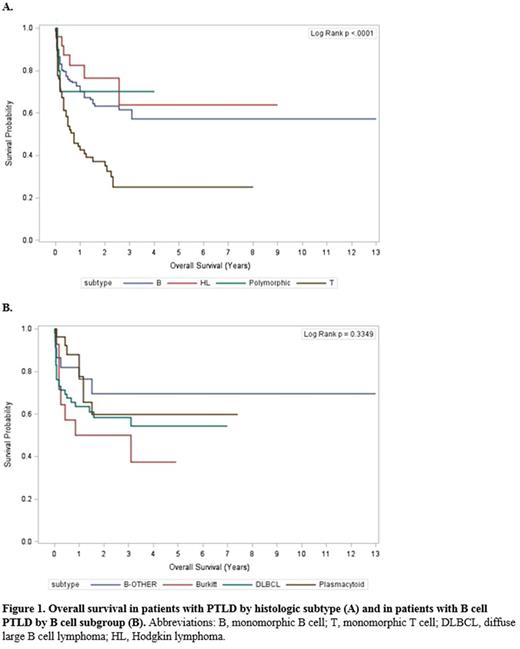
Patients with recorded subtype information were included in survival analysis. Kaplan-Meier analysis compared overall survival (OS) for four major subtypes (polymorphic, monomorphic B cell, monomorphic T cell, and Hodgkin-type neoplasms), and for four subgroups of B cell neoplasms. Univariate and multivariable Cox proportional hazard regression analyses were performed to identify predictors of OS for each subtype and B cell subgroup.
Results
Of 234 cases with available subtype information, 52.1% were monomorphic B cell neoplasms, of which DLBCL was the most frequent subtype (n=59, 48.3% of B neoplasms); 28.6% were monomorphic T cell neoplasms, 10.6% were Hodgkin-type, and 4.3% were polymorphic. OS was significantly different between monomorphic T cell neoplasms (median 9 months) and polymorphic, monomorphic B cell, and Hodgkin-type neoplasms, for which median OS was not reached (p = 0.0001, Figure 1a). Significant differences in OS among B-subgroups were not detected, but there was a trend towards decreased survival in Burkitt-type PTLD (Figure 1b). Kidney transplant and treatment with reduction of immunosuppression were associated with increased OS in multivariable analysis in B cell neoplasms. Immunosuppression with azathioprine was associated with decreased OS in T cell neoplasms, while treatment with radiotherapy was associated with improved OS in that subtype.
Conclusions
Histologic subtype represents an important factor in PTLD prognosis, with T cell monomorphic subtype exhibiting a particularly poor OS. The possibility of lower survival in certain subsets of B cell PTLD should be explored in future studies and suggests the need for subtype-specific treatment strategies to improve outcomes.
Flowers: Onyx: Research Funding; TG Therapeutics: Research Funding; Research to Practice: Research Funding; Genentech/Roche: Consultancy, Research Funding; Prime Oncology: Research Funding; Burroughs Welcome Fund: Research Funding; OptumRx: Consultancy; Seattle Genetics: Consultancy; Bayer: Consultancy; Eastern Cooperative Oncology Group: Research Funding; Janssen Pharmaceutical: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Celgene: Consultancy, Research Funding; Millennium/Takeda: Research Funding; National Institutes Of Health: Research Funding; National Cancer Institute: Research Funding; Acerta: Research Funding; Clinical Care Options: Research Funding; Infinity: Research Funding; Spectrum: Consultancy; V Foundation: Research Funding; Abbvie: Consultancy, Research Funding; Educational Concepts: Research Funding; Gilead: Consultancy. Waller: PRA: Consultancy; National Institutes of Health: Research Funding; AMGEN: Consultancy; Cambium Medical Technologies: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Katz Foundation: Research Funding; Chimerix: Equity Ownership; Celldex: Consultancy; Coulter Foundation: Research Funding; Helocyte: Consultancy; Cerus: Equity Ownership; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.