Abstract
Background and Purpose: e14a2 (b3a2) and e13a2 (b2a2) are the two major BCR-ABL1 transcripts of CML. Our previous study (EHA 2016, abs P609) showed that patients with e13a2 transcripts received imatinib as front-line therapy had a significant higher treatment failure rate at 6 and 12 months, a lower cumulative incidence of MMR by 2 years, a higher frequency of lymphoid crisis, and a trend of inferior progression-free survival (PFS) as compared to those with e14a2 patients. The prognostic relevance of the two transcript subtypes following front-line second generation tyrosine kinase inhibitors (TKIs) therapy remains to be determined. We aimed to compare the molecular responses between e14a2 and e13a2 transcripts and their impact on outcomes of CML patients treated with front-line nilotinib or dasatinib.
Patients and Methods: Between March 2008 and April 2017, there were 260 newly diagnosed CML patients in chronic phase with age older than 18 years, received 2nd generation TKIs as front-line therapy. BCR-ABL1 transcripts included 170 e14a2, 67 e13a2, 19 both e14a2 and e13a2, 2 e1a2, and one each of e14a3 and e13a3. The 237 patients carrying either e14a2 or e13a2 transcripts but not both were enrolled in this study, 143 patients received nilotinib and 94 patients received dasatinib. The e14a2 subtype accounted for 76.9% (n = 110) in the nilotinib-treated group and 63.8% (n = 60) in the dasatinib-treated group. The BCR-ABL1 levels expressed as International Scale (IS) were measured by TaqMan RQ-PCR assay with the lab-specific conversion factor every 3 months in a central laboratory.
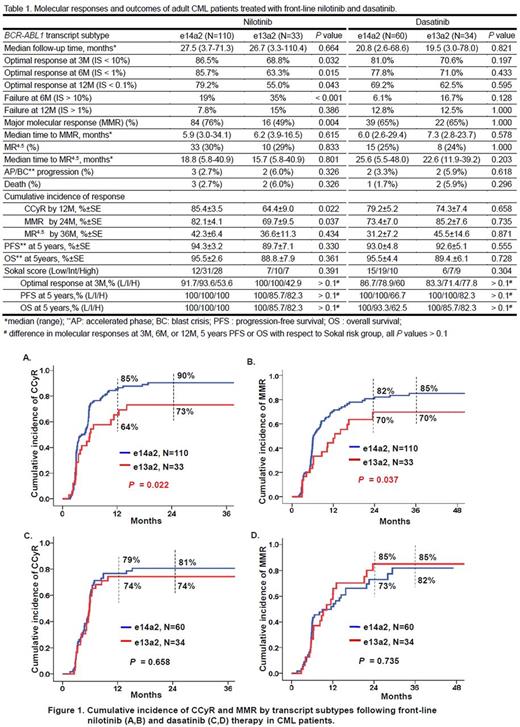
Results: Of the 237 patients with e14a2 or e13a2 transcripts, there was no difference in age, gender and Sokal score between the two transcript subtypes in each treatment group. In the nilotinib-treated group, patients with e14a2 achieving optimal molecular responses based on the ELN definition at 3, 6, and 12 months were significantly higher than those with e13a2 subtype, and had a significantly lower failure rate at 6 months (P < 0.001). In the dasatinib-treated patients, no difference was observed between the two transcript subtypes in the molecular responses at these early landmark time points. The median time to MMR or MR4.5 wasnotdifferent between the two transcript subtypes in both treatment groups. With a median follow-up of 27.5 months in the nilotinib-treated patients, 76% of e14a2 patients achieved MMR compared with 49% of e13a2 patients (P= 0.004) whereas no difference was observed for the frequency of MR4.5 between the two subtypes; 5 patients had disease progression and died. With a median follow-up of 20.8 months in the dasatinib-treated patients, there were no differences in MMR and MR4.5 rates, disease progression occurred in 4 patients and 3 of them died. In patients treated with nilotinib, the cumulative incidence of CCyR by 12 months and MMR by 24 months were significantly lower in e13a2 subtype compared with e14a2 subtype (P= 0.022, Fig. 1A and P= 0.037, Fig. 1B, respectively) but no difference in the cumulative incidence of MR4.5 between e14a2 and e13a2 by 36 months (P= 0.434). There were no difference in the cumulative incidence of CCyR by 12 months (P= 0.658, Fig. 1C), MMR by 24 months (P= 0.735, Fig. 1D) and MR4.5 by 36 months (P= 0.871) between the two transcript subtypes in the dasatinib-treated group. PFS and overall survival (OS) were not different between the two transcript subtypes in both nilotinib- and dasatinib-treated groups. Sokal risk group did not influence molecular responses, PFS or OS with respect to transcript subtypes in both treatment groups. The molecular responses and outcomes of e14a2 and e13a2 CML patients treated with front-line nilotinib and dasatinib are summarized in Table1.
Conclusions: The results of Taiwan CML study demonstrated that patients with e13a2 transcripts had a significantly inferior early molecular responses compared with e14a2 when treated with front-line nilotinib. There was no difference in molecular responses between the two transcripts in patients treated with dasatinib. Sokal risk group had no impact on the treatment responses, irrespective of transcript subtypes.
Shih: Novartis (Taiwan) Co., Ltd.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.