Abstract
Background:
Myeloproliferative neoplasms (MPN) including polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF) are clonal hemopathies characterized by burdensome symptom profiles and impaired quality of life. Treatments include pharmacologic approaches, phlebotomy, and bone marrow transplant. Outcome studies have historically focused on hematologic improvement and survival benefit. Few prospective studies have evaluated patient-reported symptoms and quality of life outcomes among standard pharmacologic and medical therapies. The Myeloproliferative Neoplasm Quality of Life (MPN-QOL) Study Group aims to objectively quantify symptomatic response to standard treatments. Here we provide updated results for a prospective trial: the MPN Experimental Assessment of Symptoms by Utilizing Repetitive Evaluation (MEASURE) trial.
Methods:
The MEASURE trial is a prospective international cohort study evaluating responsiveness of the MPN-SAF TSS to changes in target symptoms for an anticipated 480 ET, PV, and MF patients receiving non-experimental medical therapy and/or phlebotomy.
Patients complete the Myeloproliferative Neoplasm Symptom Assessment Form - Total Symptom Score (MPN-SAF TSS; JCO 2012) for seven consecutive days at enrollment then again for seven consecutive days between 90 days and six months later. Patients also complete the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 (EORTC QLQ-C30) and M.D. Anderson Symptom Inventory (MDASI) instruments at enrollment and on the first day of the second assessment. Physicians acquire demographic, laboratory, physical examination, and radiographic data.
Results:
Demographics
To date, 120 patients have been enrolled and 82 have completed both study visits. Participants include ET (67%), PV (18%), and MF (15%; including 60% primary MF and 40% post-PV) patients. Enrolled patients are 54% female. The most common therapies received prior to enrollment were aspirin (39%), hydroxyurea (20%), phlebotomy (17%), and warfarin/clopidogrel/anticoagulation (6%). The most common current MPN therapies were hydroxyurea (65%), aspirin (28%), ruxolitinib (10%), and interferon (9%). Information about mutational analysis was also collected; 74% of enrolled patients have a known JAK2 V617F mutation and 3% have MPL W515 mutation.
Symptom Measures
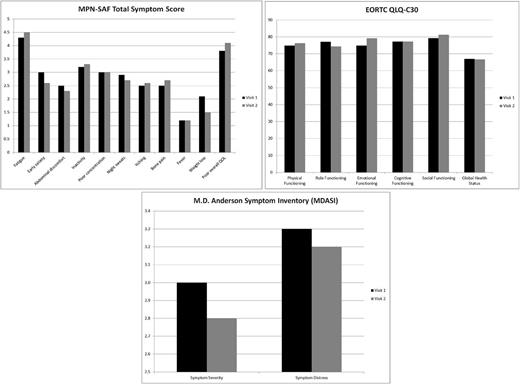
On the MPN-SAF TSS, mean scores decreased from the first to the second visit for early satiety (-0.4), abdominal discomfort (-0.2), night sweats (-0.2), and weight loss (-0.6). Mean scores increased for fatigue (+0.2), inactivity (+0.1), itching (+0.1), bone pain (+0.2), and overall poor quality of life (+0.3). There were no changes for concentration problems or fevers. Cumulative MPN-SAF TSS score decreased from a mean of 27.1 to 26.3. On the QLQ-C30, mean scores improved for physical (+1.4), emotional (+4.3), and social functioning (+1.9) and decreased for role functioning (-2.7). There was no change for cognitive functioning. The global health status measure declined from 67.0 to 66.7 (-0.3). On the MDASI, the symptom severity score decreased from 3.0 at the first visit to 2.8 at the second (-0.2) and the symptom distress measure decreased from 3.3 to 3.2 (-0.1). See figures for scores by visit.
Discussion:
Myeloproliferative neoplasms are associated with burdensome symptoms that significantly compromise quality of life. To date, no studies have quantified symptomatic response to standard-of-care treatments including medication, phlebotomy, and bone marrow transplant. Interim results from the MEASURE trial demonstrate variable effects of treatment on disease-related symptoms on multiple patient-reported survey instruments including the MPN-SAF TSS, EORTC QLQ-C30, and MDASI.
Vannucchi: Shire: Speakers Bureau; Novartis: Honoraria, Speakers Bureau. Tam: Abbvie: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Janssen Cilag: Honoraria, Research Funding. Radia: Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Educational Grant. McMullin: Italfarmaco S.p.A.: Consultancy; Shire, Novartis: Honoraria, Speakers Bureau. Mesa: Novartis Pharmaceuticals Corporation: Consultancy; Promedico: Research Funding; Galena Biopharma, Inc.: Consultancy; Ariad: Consultancy; Celgene Corporation: Research Funding; Incyte Corporation: Research Funding; Gilead Sciences, Inc.: Research Funding; CTI BioPharma Corp.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.