Abstract
Background: EFS at 24 months (EFS24) in newly diagnosed patients with DLBCL following first line rituximab containing anthracycline-based chemotherapy has been shown to be a surrogate marker for long-term OS. In patients achieving EFS24, subsequent OS is nearly equivalent to that of the general population, suggesting that these patients will have a near normal life expectancy if they have not experienced disease relapse by this time point. (Maurer et al. JCO 2014, Jakobsen et al. JCO 2017). In this study, we aim to determine the value of EFS as a surrogate endpoint for OS among patients with relapsed and refractory de novo or transformed DLBCL undergoing ASCT.
Methods: Patients with de novo or transformed DLBCL undergoing ASCT in the CCTG LY.12 trial (Crump et al. JCO 2014) were included in the analysis. This multi-center study enrolled patients from August 2003 to November 2011. Analyses were conducted on the updated locked database of June 30th, 2017. Data for an age and sex-matched control group were taken from the Statistics Canada (http://www.statcan.gc.ca) life tables, which provide the survival rate of the general Canadian population. EFS was defined as time from ASCT until relapse, progression, initiation of new lymphoma therapy, or death from any cause. Standardized mortality ratio (SMR) was calculated for each subgroup of patients achieving EFS0 (date of ASCT) to EFS72, with increments of 12 months. All statistical calculations were performed using the SAS version 9.2 and R version 3.3.2.
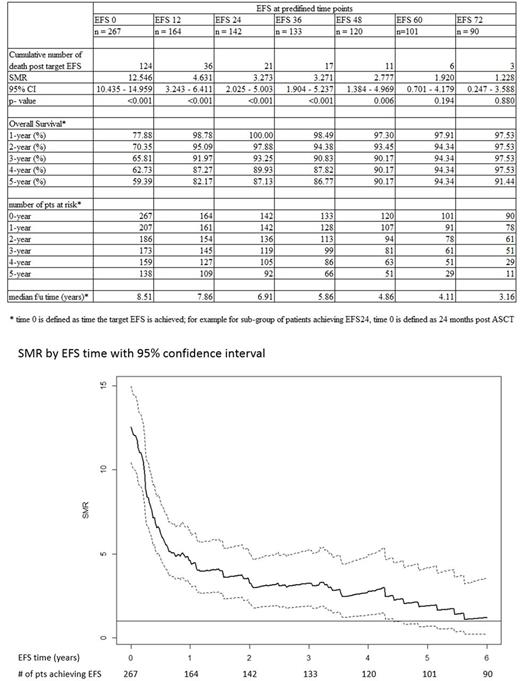
Results: A total of 267 patients were included. The mean age at study entry was 54 years (range 24 to 70); 68% were age <60 years; 61% male; relapse IPI 0-1: 43.8%, 2: 31.5 %, >3: 24.7%; de novo DLBCL 82.8%. Sixty-seven percent of patients received prior rituximab; 70% received rituximab as part of salvage chemotherapy (gemcitabine, cisplatin, dexamethasone or dexamethasone, cytarabine, cisplatin). At a median follow-up of 8.5 years, the total recorded number of deaths was 124; 59 occurred within the first year of follow up, 21 occurred among patients achieving EFS 24, and 6 occurred in patients achieving EFS60. The SMR decreased with each year of follow up, at EFS0 it was 12.55 (95% CI 10.44-14.96), 3.27 (95% CI 2.03-5.00) at EFS24, and 1.92 (95% CI 0.70-4.18) at EFS 60 (Table and Figure). The incidence of disease progression also decreased with each year of follow up, with 88 occurring within the first year of follow up, 21 within year 2, and only 3 after 5 years. More deaths were caused by non-lymphoma conditions, including second malignancy, for patients achieving EFS24 or greater. The 5-year OS was 59.4% for patients at EFS0, 87.1% at EFS24, and 94.3% at EFS60. A multivariate analysis including age at ASCT, IPI, de novo vs transformed DLBCL, and response to prior therapy did not reveal any predictors of survival once patients had achieved EFS24 or EFS60.
Conclusion: This study demonstrates that the SMR decreases over time among patients with relapsed DLBCL undergoing ASCT, and remains significant until these patients have achieved EFS60. Thus, earlier EFS cannot be used as a surrogate marker of OS in this clinical context. A larger sample size and a validation study are needed to establish the predictive value of EFS60 for OS following ASCT.
Hay: Celgene: Research Funding; Novartis: Research Funding; Janssen: Research Funding; Amgen: Research Funding; Kite: Research Funding; Abbvie: Research Funding; Roche: Research Funding. Crump: Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen Ortho: Consultancy, Membership on an entity's Board of Directors or advisory committees. Baetz: Merck: Honoraria; Novartis: Honoraria; Roche: Honoraria. Robinson: Abbvie: Honoraria; Lundbeck: Honoraria; Janssen: Honoraria; Roche: Honoraria; Gilead: Honoraria. Assouline: Bristol Myer Squibb: Speakers Bureau; Pfizer: Speakers Bureau; Novartis Canada Inc.: Honoraria; Paladin: Speakers Bureau; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.