Abstract
Introduction: Latent hepatitis B virus (HBV) infection is a well-recognized risk factor for viral reactivation and life-threatening hepatitis during chemotherapy. Chronic HBV carriers, identified by the presence of hepatitis B antigen (HBsAg) in their serum, have a well-established high risk for reactivation. Patients, exposed to HBV, who have not become true carriers [HBsAg-negative, but anti-hepatitis B core antigen (HBc)-positive] are usually considered to have a "resolved" HBV infection. Current recommendations state that during chemotherapy all patients exposed to HBV in the past are to receive anti-HBV prophylaxis. However, these recommendations are based on data mainly driven from unselected series of cancer patients previously exposed to HBV. It has been clearly demonstrated that HBV reactivation is common in cases where immune suppressive therapy is applied (e.g., patients treated with corticosteroids and/or rituximab or those undergoing stem cell transplantation). Information regarding the incidence and risk factors of HBV reactivation in patients treated for acute myeloid leukemia (AML) is scarce. While some data support prophylaxis for chronic HBV carriers (HbSAg-positive) during chemotherapy for AML, it remains debatable whether this approach is required in AML patients with "resolved" HBV infection undergoing intensive chemotherapy.
Methods: The current study reviewed medical files of 1200 patients who were diagnosed with AML between the years 2000-2017 and treated in three large hematology departments in Israel - at the Rambam Health Care Campus, Tel Aviv Sourasky Medical Center and Shaare Zedek Medical Center. Clinical characteristics and laboratory data were retrospectively analyzed. Each patient, diagnosed with AML, was tested for HbSAg, HbSAb, anti-HBc, anti-hepatitis C virus (HCV). Only patients who had not received prophylaxis with anti-HBV agents were included in the analysis.
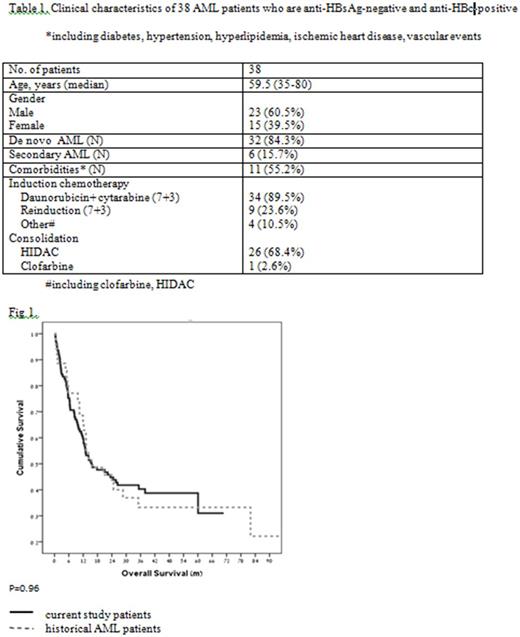
Results: A total of 38 patients with "resolved" HBV infection at the time of leukemia diagnosis were identified, including 15 females (39.5%) and 23 males (60.5%), with a median age of 59.5 years (range 35-80). Six (15.7%) patients were diagnosed with AML secondary to myelodysplastic syndromes (MDS). The following induction regimens were used: daunorubicin + cytarabine ("7+3") in 34 patients (89.5%), clofarabine in two patients, and high-dose cytarabine (HIDAC) in two patients. Nine patients received re-induction with "7+3". Twenty seven patients (71%) received consolidation - 26 with HIDAC and one with clofarabine (Table 1). Within 90 days following induction + consolidation therapy, no HBV reactivation was documented in any of the 38 patients. There was a mild and asymptomatic elevation in the levels of hepatocellular liver enzymes (up to 2.5 times the upper limit of normal) in some patients, which could also be attributed to other causes, such as drugs, sepsis, etc. In our cohort of patients, overall survival (OS) was identical to that observed in historical AML patients from our medical centers who were not HBV carriers (Fig. 1).
Conclusions: AML patients with previous HBV infection (HBsAg-negative and anti-HBc-positive) undergoing intensive chemotherapy are not at an increased risk for virus reactivation. In the current study, none of the 38 patients had HBV reactivation, suggesting that omission of anti-HBV prophylaxis during induction and consolidation chemotherapy could be considered in select AML patients. Notably, two groups of AML patients with a higher risk of HBV reactivation, i.e., recipients of allogeneic stem cell transplantation and HbSAg-positive patients, were not included in the present study and the conclusions drawn are not applicable to these patient subsets. Larger studies are warranted to explore the role of HBV prophylaxis in "silent" carriers.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.