Abstract
Introduction: Follicular Lymphoma (FL) is the second most frequent lymphoma subtype, with a rapidly increasing incidence rate. Progression of disease within two years of initial chemoimmunotherapy, experienced by ~20% of FL patients, has been shown to be a significant risk factor for poor health outcomes. Currently, there is no standard of care for treatment of these high-risk patients.
Data: Using Surveillance, Epidemiology, and End Results (SEER)-Medicare database, we identified 5234 patients who were diagnosed between 2000 and 2009. The selected patients either received second line therapy within two years of their initial therapy (~70%) or received no further therapy after their initial therapy (~30%). The primary variables of interest were the receipts of first-, second- and third-line treatments, and the duration of overall survival (OS). Treatments were categorized into four main groups as follows: (i) rituximab (R), (ii) R-cyclophosphamide and vincristine (R-CVP), (iii) R-cyclophosphamide, hydroxydaunorubicin, and vincristine (R-CHOP), and (iv) other R-containing regimens (R-Other). Patient age, sex, race, geographic region, marital status, year of diagnosis, and census tract-level characteristics such as education level and urban status were identified from the SEER data. Other predictors available in the data were FL histology, stage, presence of B-symptoms, history of anemia, extranodal primary site of involvement, Charlson comorbidity index and performance status.
Methods: We developed a multi-state model to study the clinical course of FL under first, second and third line treatments. The Aalen-Johansen estimator, a generalization of Kaplan-Meier estimator, was used to estimate the likelihood of being in one of four health states: alive following first-, second-, third-line treatment and dead. A multivariable Cox proportional hazards regression model was fitted to each transition between the model states to identify significant factors affecting the time and rate of each transition. Performance of each treatment is evaluated via multiple metrics such as (Aalen-Johansen) estimated survival probabilities, and (Cox) hazard ratios, proportion of deaths within two years and median overall survival durations. Furthermore, we compared the multi-state model with the conventional OS model to discuss the advantages of using a more detailed modeling approach.
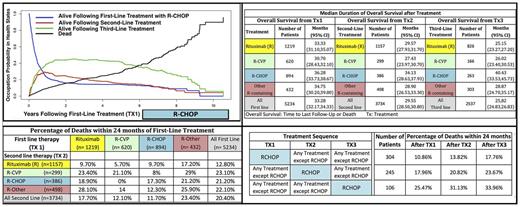
Results: R-CHOP as first line significantly improves 2- and 5-year survival probabilities. Death probabilities at 2 and 5 years of first-line treatments are as follows: 0.24 & 0.54 for R, 0.18 & 0.45 for R-CVP, 0.16 & 0.29 for R-CHOP (shown in Figure, top left), 0.26 & 0.46 for R-Other. R-CHOP in second-line and third-line also significantly improves 5-year survival probabilities. Death probabilities after 5 years of second and third-line therapies are as follows: 0.50 & 0.51 for R, 0.53 & 0.62 for R-CVP, 0.45 & 0.39 for R-CHOP, 0.53 & 0.54 for R-Other. Median OS is the highest for R-CHOP patients at any treatment line (see Figure, top right). Hazard ratios from the multivariable regression analyses are in line with these findings. R-CHOP achieves the highest positive impact if it is implemented earlier (see Figure, bottom right). However, R-CHOP followed by second-line R-CHOP yields unfavorable clinical outcomes: R-CHOP + R = 9.7%, R-CHOP + R-CVP = 8%, R-CHOP + R-CHOP = 17.3% and R-CHOP + R-Other = 12.3% death within two years (see Figure, bottom left).
Conclusion: Using SEER-Medicare data and a multi-state modeling framework, we evaluated (1) the performance of sequential treatment strategies and (2) the impact of demographic and clinical factors on the clinical outcomes for high-risk FL patients. We found that R-CHOP in any line significantly improves FL outcomes for high-risk patients. Furthermore, our results showed that using R-CHOP earlier achieves the most favorable survival. This multi-state analysis using a large cohort of elderly patients helped identify the order in which interventions have the greatest impact and the factors that lead to worse outcomes for FL across the lines of therapy.
Flowers: Gilead: Consultancy; Abbvie: Consultancy, Research Funding; Janssen Pharmaceutical: Research Funding; National Cancer Institute: Research Funding; Genentech/Roche: Consultancy, Research Funding; Burroughs Welcome Fund: Research Funding; Prime Oncology: Research Funding; Acerta: Research Funding; V Foundation: Research Funding; TG Therapeutics: Research Funding; Spectrum: Consultancy; OptumRx: Consultancy; Research to Practice: Research Funding; Celgene: Consultancy, Research Funding; Infinity: Research Funding; Seattle Genetics: Consultancy; Pharmacyclics LLC, an AbbVie Company: Research Funding; National Institutes Of Health: Research Funding; Onyx: Research Funding; Millennium/Takeda: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Bayer: Consultancy; Clinical Care Options: Research Funding; Educational Concepts: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.