Abstract
Background:
Hemophilia A (HA) is caused by F8 mutations and resultant plasma deficiencies in FVIII activity. While bleeding can be arrested by infusing therapeutic FVIII proteins (tFVIIIs), ~30% of severe HA patients will develop neutralizing tFVIII antibodies (inhibitors) and become resistant to treatment. For inhibitors to develop, foreign peptides must be liberated from a tFVIII and displayed on dendritic cells (DCs) in self HLAcII molecules. Little is known about the inhibitor risk attributable to distinct isomers of individual HLAcII repertoires and different tFVIII domains.
Aims:
Determine the contributions to FVIII immunogenicity risk of the distinct 1) HLAcII isomers DP, DQ and DR, and 2) tFVIII domains A1, A2, A3, B, C1 and C2.
Methods:
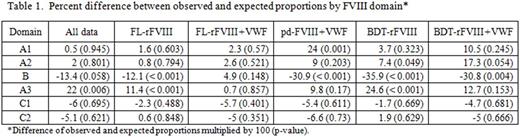
We analyzed HLAcII/tFVIII peptidomic data generated in DC-protein processing assays (PPAs) that used monocyte-derived DCs from 12 healthy donors to compare the immunogenicity of a full length (FL) and B-domain truncated (BDT) recombinant FVIII (FL- and BDT-FVIII) ± von Willebrand Factor (VWF) and a plasma derived (pd)FVIII + VWF. All tFVIIIs contain 3 small acidic-residue-rich peptides (a1, a2 and a3) that connect A1 to A2, A2 to B, and B to A3. To simplify our analysis, any LC-MS/MS individually sequenced peptide (ISP) with an N-terminal residue in either the (i) C-terminal portion of A1, A2 or B that extends into the adjacent a1, a2 or a3 region, respectively, was placed into that domain, or (ii) a1, a2 or a3 region, whether or not it extends into A3, C1 or C2, was placed into that domain. We then calculated the expected frequency of peptides from each FVIII domain (including, when appropriate, the adjacent acidic regions) based on residue number. Finally, we compared the observed and expected peptide proportions and performed a Z-test on the difference of proportions per domain. We performed this analysis for all ISP data in which the observed frequencies were obtained by a weighting scheme jointly taking into account contributions by tFVIII & by domain (All-data). We also performed this analysis by tFVIII.
Results:
For the All-data comparisons, we found that A3 had a higher observed than expected proportion (dP = 22%; p = 0.006). For the FL-FVIII comparisons, the B domain had a lower observed than expected proportion (dP = -12.1%; p < 0.001) and A3 had a higher observed than expected proportion (dP = 11.4% p < 0.001). There were no significant differences for the FL-FVIII + VWF comparisons. For pdFVIII + VWF, we found that A1 had a higher observed than expected proportion (dP = 24%; p = 0.001) while B had a lower observed than expected proportion (dP = -30.9%; p < 0.001). For BDT-FVIII, we found that A2 and A3 both exhibited higher observed than expected proportions (dP = 7.4% & 24.6%; p = 0.049 & p < 0.001), and, as expected, that the B domain had a lower observed than expected proportion (dP = -35.9%; p < 0.001). Regarding the BDT-FVIII + VWF comparisons, we found B had a lower observed than expected proportion (dP = -30.8; p = 0.004).
We also approached these data from the HLAcII perspective. We found that our 12 donors expressed 12 distinct DR molecules based on their DRB1 alleles. After tallying the per allele density of FVIII residues (for all 2332 residues) falling in the DR-bound fraction of peptides, we constructed FVIII residue-by-allele matrices of dimensions 2332 x 12 per tFVIII and performed singular value decompositions that yielded their respective matrices of right singular vectors also known as principal components (PCs). Such PCs are said to span or generate a corresponding vector subspace. Using matrix analytic methods, we can compare the therapeutic-specific subspaces to see if such contrasts reflect differences in their associated density profiles. To compare two subspaces, we compute their projection onto their intersection subspace, and use this projection to compute a subspace distance. We test the null hypothesis of a subspace distance equal to 0 (for statistically similar subspaces) by a permutation test. As an example, we compared the FL-FVIII + VWF and pdFVIII + VWF subspaces, and found that they are significantly different (subspace distance (SD) = 0.96; p < 0.001). We also compared the FL-FVIII with & without VWF subspaces, and found that they are significantly different (SD = 0.545; p < 0.001).
Conclusions:
Our findings demonstrate that the distinct domains of tFVIIIs and different alleles of individual HLAcII repertoires differentially contribute to inhibitor risk.
Hofmann: CSL Behring: Employment. Escobar: Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Shire: Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees; NovoNordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dinh: Haplomics: Employment. Powell: CSL Behring: Employment. Howard: CSL Behring: Honoraria, Research Funding; Haplomics: Employment, Equity Ownership, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.