Abstract
Background The most significant complication in the management of patients with severe hemophilia A continues to be the development of anti-factor VIII antibodies. The standard of care for inhibitor eradication is immune tolerance induction (ITI). ITI refers to frequent and prolonged exposure to fVIII, often at high doses, as a method to induce tolerance. Patients with severe hemophilia A typically develop a polyclonal B-cell response to fVIII, with antibodies to the A2 and C2 domains being the most prevalent. We have previously identified 5 non-overlapping B cell epitopes with unique characteristics in the A2 domain, and 3 in the C2 domain. Specifically within the A2 and C2 domain we have shown that epitope is a better predictor of response to treatment than the Bethesda titer. Using monoclonal antibodies (MAbs) with known epitopes, we tracked the change in A2 and C2 epitope response over time in 9 high-titer inhibitor patients during ITI.
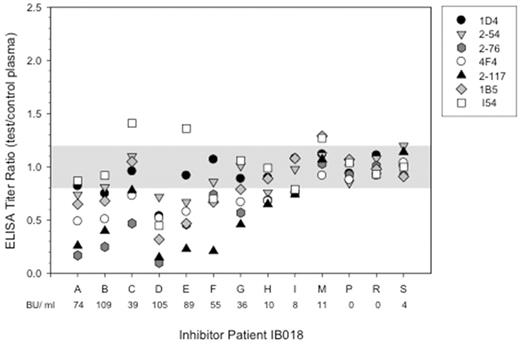
Methods B-cell epitopes were mapped using a previously published competition ELISA. For each epitope tested, a single well characterized biotinylated anti-fVIII MAb was competed against antibodies present in patient plasma. FVIII deficient plasma was used as a no antibody control. In the A2 domain, 4 of the 5 non-overlapping epitopes were tested (groups A, B, D, and E). All three non-overlapping epitopes in the C2 domain were tested (A, B, and C). Absorbance was measured at 405 nm and ELISA titers were calculated using an O.D. value of 0.3. Samples were considered positive if there was at least a 20% decrease in the ELISA titer ratio between patient and control plasma.
Results We tested 85 plasma samples from 9 patients, with each individual patient contributing between 4-13 samples over the course of ITI. At the point of highest titer, patients had a mean of 4.4 of 7 tested epitopes. The most frequently detected epitopes were 2-76 (group A) and 4F4 (group B) in the A2 domain and 2-117 (group C) in the C2 domain. All patients (9 of 9) had antibodies to these epitopes at one or more time points during ITI. The 1D4 (group E) and 2-54 (group D) epitopes within the A2 domain and the I54 (group A) and 1B5 (group B) epitopes within the C2 domain were less frequent but still prominent, with 8 of 9 patients having the I54 epitope, 7 of 9 having the 1D4 epitope, and 6 of 9 having the 2-54 and 1B5 epitopes.
Eighty of the 85 patient samples tested were found to have a positive Bethesda titer, indicating inhibitory antibodies were present. Higher inhibitory titers correlated with more epitopes detected. Of the 80 inhibitory samples, 64% of them showed antibodies to the 2-76 epitope, 59% to the 4F4 epitope, and 53% of them showed antibodies to the 2-117 epitope. The less frequent 1D4, 2-54, I54, and 1B5 epitopes were seen in 28%, 18%, 23%, and 16% respectively. The number of epitopes and the titers for those epitopes detected increased or decreased with the Bethesda titer over time. However, the most prevalent epitopes remained dominant even at times of relapse.
Conclusion A broad antibody spectrum was seen in patients with hemophilia A and inhbitors. Multiple different epitopes within the A2 and C2 were present. Given our previous studies that showed epitope was more important than titer in the pathogenicity of these antibodies, tracking epitopes over time may provide valuable knowledge regarding response to therapy and may direct the clinician to adjust ITI strategy. Ultimately, knowledge of the dominant epitope that persists or recurs after ITI may be a target for individualized treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.