Abstract
Introduction: Acute promyelocytic leukemia (APL) often presents with pancytopenia and disseminated intravascular coagulopathy and usually associated with a fusion of the promyelocytic leukemia (PML) gene on chromosome 15 with the retinoic acid receptor alpha (RARa) gene on chromosome 17 due to a chromosomal translocation t(15;17). All-trans retinoic acid (ATRA) is the standard of care for the initial emergent management of APL due to its ability to reverse the associated potentially life-threatening coagulopathy. Early death rates (EDR), defined as death in the first 30 days from APL presentation are less than 10% in clinical trials but are 12-24% in registry studies. ATRA plus arsenic trioxide (ATO) chemotherapy is highly effective and leads to cure in over 90% of cases, but differentiation or cytokine release syndrome (DS) is a potentially life-threatening complication. The availability and early use of ATRA when APL is suspected may lead to a decrease in the EDR and is considered an important treatment quality measure. We performed a retrospective chart review to determine if the timing of initial ATRA administration affects the development of DS symptoms and EDR.
Methods: In this study, patients who received ATRA within 24 hours of presentation to the hospital were considered to have early ATRA administration. Differentiation syndrome criteria included: dyspnea; unexplained fever; weight gain >5kg; unexplained hypotension; acute renal failure; and pulmonary infiltrate/pleural effusion. Moderate DS was defined as having 2 or 3 symptoms and severe DS was defined as having 4 or more symptoms. Patients received dexamethasone for treatment of DS based on severity of symptoms. Patient and clinical characteristics were summarized as numbers and percentages for categorical variables, median and range for continuous variables. Association between timing to ATRA administration baseline characteristics and development of DS were summarized using Fisher's exact test for categorical variables and Wilcoxon's rank sum test for continuous variables.
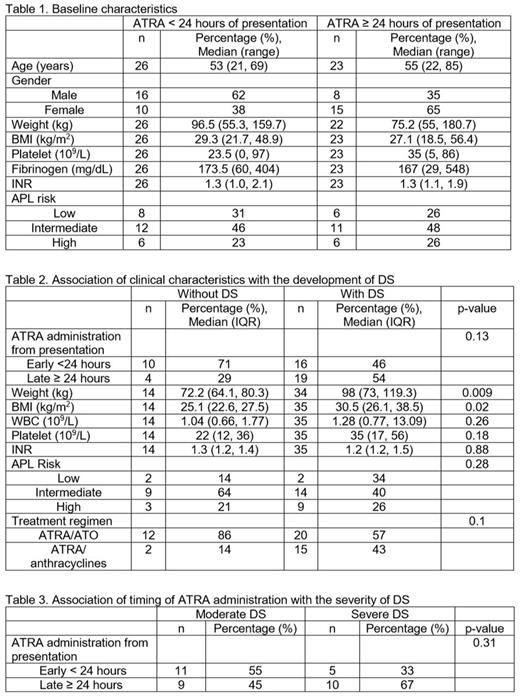
Results: Forty-nine patients who received a dose of ATRA for APL at DFCI/BWH from May 2009 - October 2016 were included in the study. There were no differences in baseline characteristics for patients based on time to ATRA administration (Table 1). Mean body mass index (BMI) was higher in patients who developed at least 2 symptoms of DS (30.5 kg/m2) than those who did not (25.1 kg/m2; p=0.02). There were no differences in the development of DS based on time of ATRA administration (p=0.13), APL risk (p=0.28), baseline WBC counts (p=0.26), platelet (p=0.18), fibrinogen (p=0.86) and INR (p=0.88) levels and the administration of ATRA plus ATO- vs. ATRA plus anthracycline-based regimens (p=0.1) (Table 2). Timing of ATRA administration had no association with the development of moderate or severe DS (p=0.31) (Table 3) or time to developing DS (p=0.99). Twenty-one patients received treatment for DS with dexamethasone at the discretion of the treating physician. One patient died during the first 30 days due to intracranial hemorrhage.
Conclusion: The timing of ATRA administration did not significantly affect the rates or timing of developing clinical symptoms of DS, however a large study is warranted. Early identification and treatment of DS is essential for APL patients during induction, and should be considered even more strongly in patients with elevated BMI. Our low EDR compared to previous studies may indicate recent advancements in the awareness, diagnosis and treatment of APL.
McDonnell: Spectrum: Membership on an entity's Board of Directors or advisory committees. Stone: Abbvie: Membership on an entity's Board of Directors or advisory committees; Arog: Membership on an entity's Board of Directors or advisory committees, Research Funding; Otsuka: Membership on an entity's Board of Directors or advisory committees; Orsenix: Membership on an entity's Board of Directors or advisory committees; Cornerstone: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Argenix: Other: DSMB; Actinium: Membership on an entity's Board of Directors or advisory committees; Fujifilm: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sumitomo: Membership on an entity's Board of Directors or advisory committees; Seattle genetics: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Merck: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Ono: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Jazz: Membership on an entity's Board of Directors or advisory committees. DeAngelo: Takeda Pharmaceuticals U.S.A., Inc.: Honoraria; Shire: Honoraria; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; Glycomimetics: Research Funding; Pfizer Inc.: Consultancy, Honoraria, Research Funding; BMS: Consultancy; Blueprint Medicines: Honoraria, Research Funding; ARIAD: Consultancy, Research Funding; Immunogen: Honoraria, Research Funding; Amgen: Consultancy, Research Funding; Incyte: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.