Abstract
Background and Purpose
Chronic myeloid leukemia (CML) is a rare disease in children and constitutes 2% to 3% of pediatric leukemia. Imatinib, a potent tyrosine kinase inhibitor (TKI), is well established as a standard treatment for newly diagnosed adults with CML in chronic phase. Many pediatric oncologists have followed the treatment guidelines that originally designed for adult patients. There have been rare studies reporting the molecular responses to imatinib in children with CML. The aim of this multi-center study in Taiwan was to assess the treatment responses and outcomes of front-line imatinib therapy in pediatric CML. A comparison between pediatric and adult CML patients was also performed.
Patients and Methods
Between 2002 and 2017, there were 53 newly diagnosed children, age < 18 years old, with CML in chronic phase receiving front-line imatinib therapy with a daily dosage of 300 mg to 400 mg. b3a2, b2a2, b3a2+b2a2, and b3a3 BCR-ABL1 transcripts were detected in 32, 19, 1 and 1 patient(s) respectively. Fifty-two patients with b3a2 and or b2a2 were enrolled in this study. Peripheral blood BCR-ABL1 levels expressed as International Scale (IS) were measured by TaqMan RQ-PCR assay with the lab-specific conversion factor every 3 months in a central laboratory. The criteria of the European LeukemiaNet regarding optimal response were adopted: major cytogenetic response (MCyR, IS < 10%) at 3 months, complete cytogenetic response (CCyR, IS < 1%) at 6 months and major molecular response (MMR, IS < 0.1%) at 12 months (Blood 2013). Molecular response of a patient was censored if the patient received hematopoietic stem cell transplantation (HSCT), switched to 2nd-generation TKI, or died. An adult cohort of 685 patients who had molecular measurements in the same central laboratory, presented partly in ASH 2014 (Blood 2014. 124: 4544a), was updated for the comparison analysis.
Results
Of the 52 pediatric CML patients, 31 were males, the median age at diagnosis of 14.2 years (range, 1.4-17.9). The WBC count ranged from 24.5 to 806.5×109/L (median, 294.4). High Sokal risk group was only observed in 4% and 14.6% for score adopted for younger than 45 years old, respectively. Eight patients went on to receive HSCT, and 20 patients were shifted to 2nd-generation TKIs (14 dasatinib, 6 nilotinib), including 3 achieved optimal responses because of imatinib intolerance. Compared with the adult cohort, there was no statistical difference in the frequency of transcript subtypes. Pediatric patients classified as Sokal high-risk group were significantly lower than those in adult cohort (P < 0.0001).
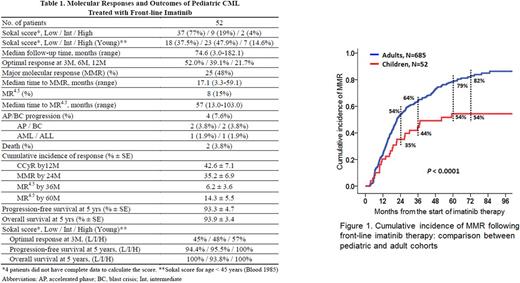
The molecular responses and outcomes of the pediatric cohort are summarized in Table 1. The median followed-up duration was 74.6 months (range, 3.0-182.1). Optimal responses at 3 months, 6 months and 12 months following imatinib therapy were achieved in 52.0%, 39.1% and 21.7%, respectively. MMR and MR4.5 (IS ≤ 0.0032%) were achieved in 48% and 15% of pediatric patients, respectively. The cumulative incidences (% ± SE) of CCyR by 12 months, MMR by 18 months and MMR by 24 months were 42.6 ± 7.1, 26.5 ± 6.3 and 35.2 ± 6.9, respectively. The cumulative incidence of MMR in pediatric patients was inferior to that in adult cohort (P < 0.0001) (Figure 1). Progression to accelerated phase and blast crisis occurred in 2 patients each. The 10-year progression-free survival (PFS) and overall survival (OS) (% ± SE) were not significantly different between pediatric and adult cohorts (90.4 ± 4.7 vs. 93.0 ± 1.3, P= 0.533; 90.6 ± 4.7 vs. 94.3 ± 1.6, P= 0.176).
The IS levels at 3 months among three different Sokal risk groups did not differ in pediatric CML. Patients achieving MMR in Sokal low-risk group of pediatric cohort were significantly lower than those of adult patients (P= 0.002) whereas the PFS and OS by Sokal risk group in pediatric and adult CML patients did not show statistical difference.
Conclusions
The molecular responses in pediatric CML patients receiving front-line imatinib therapy were inferior to those of adult cohort, but the PFS and OS did not differ between the two cohorts. The Sokal scores did not predict outcome in pediatric CML.
Shih: Novartis (Taiwan) Co., Ltd.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.