Abstract
Introduction: ASXL1 gene mutations commonly occur in myeloid diseases and are associated with inferior prognoses. They can occur as frameshift, nonsense or missense mutations. In myelofibrosis (MF) specifically, ASXL1 mutations are associated with worse overall survival (OS) and leukemia-free survival (LFS). While the general impact of ASXL1 mutations has been well-studied, the variable impact based on type of mutation is less clear. In CMML and MDS, frameshift and nonsense mutations correlate with shorter survival and increased risk of leukemic transformation. In this study, we aimed to analyze the role of ASXL1 mutations in MF while stratifying for type of mutation.
Methods: We retrospectively analyzed MF patients seen at our institution who had next-generation sequencing (NGS) performed as part of routine clinical care between 9/1/2013 and 5/1/2017. Laboratory and clinical parameters calculated from first presentation. OS defined from time of NGS assessment.
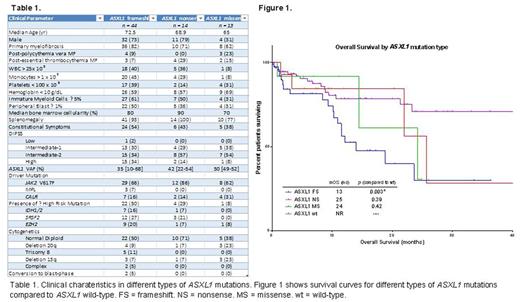
Results: We evaluated 182 MF patients with available molecular profiling. ASXL1 mutations were present in 71 (39%) patients. Among ASXL1 mutations, 44 were frameshift (FS) mutations (62%), 14 were nonsense (NS) mutations (20%) and 13 were missense (MS) mutations (18%). Demographic and clinical features of these groups can be seen in figure 1. Grouped together, ASXL1 mutations were associated with high-risk clinical features such as decreased hemoglobin (p = 0.003), decreased platelet count (p = 0.01), increased bone marrow fibrosis (p = 0.02), transfusion dependence (p = 0.005), increased LDH (p = 0.02), and higher MF-specific prognostic scores (IPSS, DIPSS, and DIPSS+) (p < 0.001, p < 0.001, p = 0.003, respectively). Excluding MS mutations, the correlation with high-risk features was strengthened and new correlations between ASXL1 mutations and leukocytosis (p = 0.006), monocytosis (p = 0.02), and the presence of EZH2 (p = 0.04), NRAS (p = 0.008), and SRSF2 (p = 0.01) mutations emerged. ASXL1 NS mutations were associated with presence of splenomegaly (p = 0.01), increased immature myeloid cells (p = 0.03), and high IPSS (p = 0.04) and DIPSS (p = 0.04) scores when compared to ASXL1 wild-type (WT) patients. ASXL1 MS mutations correlated with decreased hemoglobin (p = 0.03) and decreased bone marrow cellularity (p = 0.007), but did not correlate with prognostic scores.
High molecular risk (HMR) mutations (SRSF2, EZH2, IDH1/2) occurred more commonly with ASXL1 FS mutations than with ASXL1 MS mutations (p = 0.003) or ASXL1 WT (p = 0.002). SRSF2 mutations occurred more commonly in patients with ASXL1 FS mutations compared to ASXL1 MS mutations (p = 0.02) and ASXL1 WT (p = 0.03). EZH2 mutations occurred more commonly with ASXL1 FS mutations than with ASXL1 WT (p = 0.02).
Including all ASXL1 mutations, there was a trend towards inferior LFS (p = 0.12) when compared to ASXL1 WT. Excluding NS and MS mutations, ASXL1 FS mutations were associated with decreased LFS (p = 0.02). The rate of transformation to blast-crisis was 14% with FS mutations, 0% with NS and MS mutations, and 4% in ASXL1 WT patients. ASXL1 mutations were associated with inferior OS (p = 0.005). When assessed by type of mutation, only FS mutations were associated with inferior OS (p = 0.003). NS mutations were not associated with OS (p = 0.39).
Conclusion: The impact of ASXL1 mutations on clinical phenotype and outcome is most clearly demonstrated with frameshift mutations. Nonsense mutations appear clinically significant given their correlation with phenotype and higher-risk disease, but their impact on survival is less clear. Missense mutations do not have an apparent impact in MF. Increased presence of additional high-risk mutations in patients with ASXL1 frameshift mutations suggests a more unstable genome and likely contribute to inferior outcomes.
Sallman: Celgene: Research Funding. Padron: Incyte: Honoraria, Research Funding. Lancet: Pfizer: Other: Institutional research funding; Biopath, Biosight, Boehringer Ingelheim, Celator/Jazz, Celgene, Janssen, Karyopharm Therapeutics, and Novartis: Consultancy. Komrokji: Celgene: Honoraria; Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.