Abstract
Introduction: Patients with multiple myeloma (MM) experience clinical manifestations of hypercalcemia, renal insufficiency, anemia, or bone lesions. The National Comprehensive Cancer Network (NCCN) recommends specific testing as part of the initial workup of patients suspected of having MM. Connect MM is the first and largest US-based, multicenter, prospective observational registry of patients with newly diagnosed MM (NDMM). This registry includes patients with NDMM treated in community, academic, and government practices, thus allowing a reasonable generalizability to diagnostic patterns for the US. Previous work characterized baseline characteristics for patients enrolled in the first cohort of the registry (Rifkin, Clin Lymph Myeloma Leuk, 2014). The primary objectives of this analysis were to describe the baseline demographic and disease characteristics and to analyze the completion of diagnostic work-up of NDMM patients enrolled from 2009 to 2016.
Methods: Connect MM (NCT01081028) was initiated in September 2009 at 250 community and academic sites. Patients were enrolled sequentially in 2 cohorts. Cohort 1 enrolled 1493 patients with NDMM from September 2009 to December 2011, and Cohort 2 enrolled 1518 patients with NDMM from December 2012 to April 2016. This current analysis was conducted in the populations of treated patients in Cohort 1 (n = 1450) and Cohort 2 (n = 1462). Baseline demographics and disease characteristics for Cohorts 1 and 2 individually were evaluated. The usage of NCCN-suggested diagnostic work-up as part of the initial diagnostic characterization of NDMM was also examined.
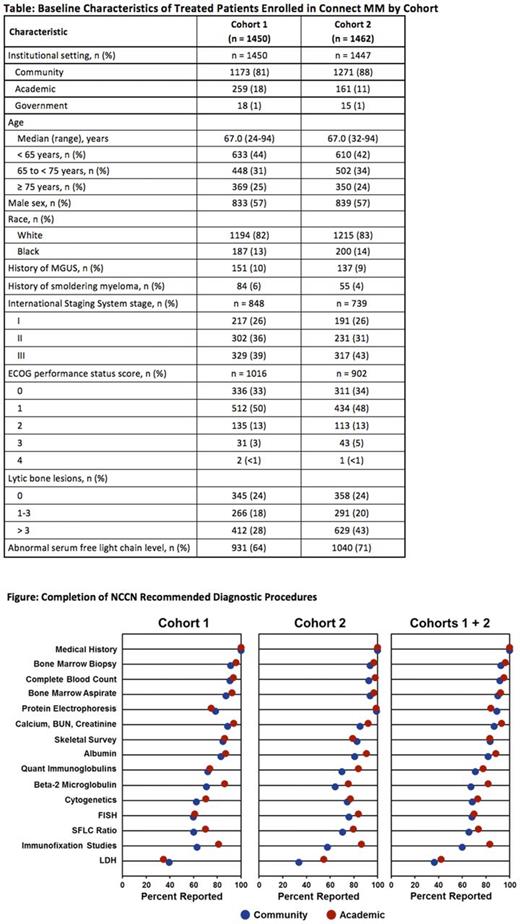
Results: Across both cohorts, most patients (84%) were treated at community sites, with some in academic (15%) and government (1%) centers. The data cutoff date was April 2017. The median follow-up for Cohort 1 is 39.3 months and for Cohort 2 is 21.4 months. Baseline characteristics are listed in the Table. Cohorts 1 and 2 were similar in age (median 67 years), sex (57% male), and race (83% white). Patients with a documented history of MGUS, smoldering myeloma, or asymptomatic myeloma were uncommon (<10%) in both cohorts. Notably, ISS staging at baseline was reported for 59% and 51% of patients in Cohorts 1 and 2, respectively. The Figure lists the completed NCCN recommended diagnostic procedures. Significant differences were detected between the two cohorts in five assessments. The assessment of β2-microglobulin was reported for 74% (1070 of 1450) of patients in Cohort 1 compared with 66% (961 of 1462) in Cohort 2. Protein electrophoresis was assessed more frequently in Cohort 2 (99%; 1447 of 1462) vs Cohort 1 (78%; 1131 of 1450). Likewise, the serum free light chain ratio was assessed more frequently in Cohort 2 (72%; 1050 of 1462) than Cohort 1 (62%; 900 of 1450). Conventional cytogenetic analysis and FISH analysis were performed more frequently in Cohort 2 than in Cohort 1: 75% vs 64% and 77% vs 60%, respectively.
Conclusions: This analysis outlines the diagnostic patterns from a real-world, multi-institutional registry of NDMM in the US. Results from this analysis indicate that the baseline demographics were largely unchanged from Cohort 1 and Cohort 2, which supports the pooling of data for future analyses. The multicenter nature of these data may better reflect clinical practice compared with single-center databases. The baseline testing of key factors required for accurate diagnosis and assessment, and adequate monitoring of patients with NDMM to guide therapy decisions is variable. Missing baseline diagnostic work-up assessments may impact ISS staging and risk stratification. Our findings suggest a better adherence to guidelines and adoption of diagnostic testing as standard of care over time.
Narang: Celgene: Consultancy, Speakers Bureau; Janssen: Speakers Bureau. Abonour: Takeda: Other: Steering committee, Research Funding; Prothena: Research Funding; Celgene: Other: Steering committee, Research Funding. Durie: Takeda: Consultancy; Janssen: Consultancy. Gasparetto: Janssen, BMS, Celgene, Takeda: Honoraria; Celgene: Research Funding; Janssen, BMS, Celgene: Consultancy; Janssen, BMS, Celgene: Other: Travel, accommodations, or other expenses paid or reimbursed. Toomey: Myriad Genetics: Speakers Bureau; Dava Oncology: Other: Travel; Celgene: Consultancy. Hardin: Celgene: Consultancy. Jagannath: Medicom: Speakers Bureau; Novartis: Consultancy; MMRF: Speakers Bureau; Celgene: Consultancy; Merck: Consultancy; Bristol-Meyers Squibb: Consultancy. Terebelo: Janssen: Speakers Bureau; Takeda: Speakers Bureau; Pharmacyclics: Speakers Bureau; Celgene: Consultancy. Wagner: EveryFit: Consultancy; Gilead: Consultancy; Janssen: Consultancy. Srinivasan: Celgene: Employment. Flick: Celgene: Employment. Zafar: Celgene Corporation: Employment. Kitali: Celgene: Employment. Agarwal: Celgene Corporation: Employment. Rifkin: Celgene: Consultancy; EMD Serono: Consultancy; Sandoz: Consultancy; Takeda: Consultancy; McKesson: Other: Stock; Boehringer Ingelheim: Consultancy; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.