Abstract
Background/Objective: Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, potentially life-threatening hematologic disorder characterized by chronic uncontrolled intravascular hemolysis, thrombosis, end organ damage, and impaired quality of life (QoL) and survival. The International PNH Registry (NCT01374360) is an ongoing, global observational study to evaluate the safety profile of eculizumab and to characterize the burden and progression of PNH including clinical outcomes, morbidity, and mortality irrespective of treatment. The objective of this analysis was to characterize the demographic and clinical characteristics, disease-associated morbidities, PNH symptoms, and health-related QoL of the largest cohort of PNH patients enrolled in the Registry.
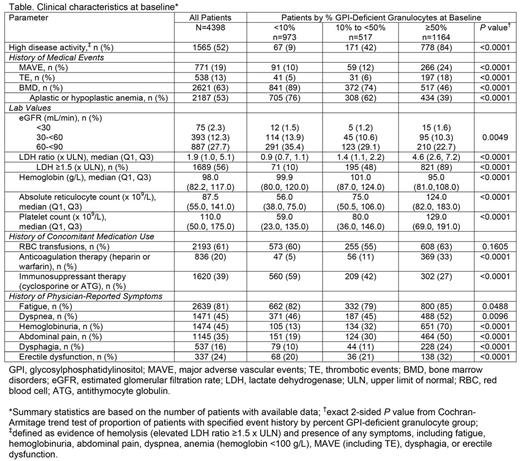
Methods: Registry patients who had data available as of May 1, 2017, were eligible for the current analysis. All patients were untreated at baseline, defined as the date of eculizumab initiation for patients ever treated with eculizumab and the date of Registry enrollment for patients never treated with eculizumab. Proportions of patients with high disease activity (HDA), history of major adverse vascular events (MAVE; including thrombotic events [TE]) and bone marrow disorders (BMD) including aplastic anemia, red blood cell (RBC) transfusions, concomitant medication use, and physician-reported symptoms were based on the number of patients with non-missing responses at baseline. Laboratory values and QoL scores assessed with the European Organisation for Research and Treatment of Cancer [EORTC] Global Health and Functional Assessment of Chronic Illness Therapy [FACIT]-Fatigue questionnaires within 6 months prior to baseline were summarized using medians and quartiles or proportions for the patients with non-missing responses. Patients with percent glycosylphosphatidylinositol (GPI)-deficient granulocyte values available prior to baseline were stratified based on the most recent value prior to baseline and statistical analysis (i.e., P values) was used to assess potential trends related to clone size.
Results: A total of 4903 patients were enrolled. Of these, 4398 had non-missing data on demographics, enrollment date, and eculizumab status, and were eligible for the analysis. Median (Q1, Q3) age at disease onset was 35.3 (23.9, 53.1) years; 53% of the patients were female. Median (Q1, Q3) percent GPI-deficient granulocyte clone size was 33% (3%, 85%) in the 2654 patients with clone size reported prior to baseline. Clinical characteristics at baseline for the overall population and stratified by percent GPI-deficient clone size are summarized in the Table. Overall, patients had a high burden of disease at baseline, as reflected by the proportion with HDA (52%), history of MAVE (19%), BMD (63%), RBC transfusions (61%), and impaired renal function defined as estimated glomerular filtration rate <90 mL/min (42%). Patients frequently experienced symptoms such as fatigue (81%), dyspnea (45%), hemoglobinuria (45%), abdominal pain (35%), and impaired QoL with median (Q1, Q3) FACIT-Fatigue and EORTC Global Health scores of 34.0 (27.0, 40.0) and 58.3 (41.7, 75.0), respectively. All measures of disease burden except for history of RBC transfusions were significantly correlated with clone size (P <0.05); specifically, higher disease burden correlated with larger clone size. However, a substantial number of patients with clone size <10% had hemolysis (LDH ≥1.5 x ULN; 10%), MAVE (10%), HDA (9%), and/or experienced symptoms such as fatigue (82%), dyspnea (46%), abdominal pain (19%), or hemoglobinuria (13%). History of RBC transfusion and immunosuppressive therapy were reported in 60% and 59% of patients with clone size <10%, potentially due to patients with history of BMD. Interestingly, median LDH ratio and the proportion of patients with history of MAVE and TE were modestly higher in the cohort with clone size 10-<50% vs <10%, while the proportion of those patients was substantially higher in the cohort with clone size ≥50% vs 10-<50%.
Conclusions: PNH is associated with high burden of disease, particularly in patients with larger GPI-deficient clone sizes; however, even patients with smaller clone sizes had significant disease burden and symptoms. Improved characterization of clinical measures and of relationships between these measures may help inform patient management.
Schrezenmeier: Alexion Pharmaceuticals, Inc.: Honoraria, Other: Research Funding to University of Ulm . Maciejewski: Ra Pharma: Consultancy; Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Speaker Fees; Apellis Pharmaceuticals: Consultancy. Roeth: Roche: Consultancy, Honoraria; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria. Araten: Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria. Kanakura: Alexion Pharmaceuticals, Inc.: Honoraria, Research Funding; Astellas: Research Funding; Bristol Myers: Research Funding; Toyama Chemical: Research Funding; Kyowa Hakko Kirin: Research Funding; Eisai: Research Funding; Shionogi: Research Funding; Fujimotoseiyaku: Research Funding; Pfizer: Research Funding; Nippon Shinyaku: Research Funding; Chugai Pharmaceutical: Research Funding. Larratt: Alexion Pharmaceuticals, Inc.: Honoraria, Other: Research Funding to the laboratory at University of Alberta, for ADAMTS13 testing. Shammo: Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding. Wilson: Alexion Pharmaceuticals, Inc.: Employment, Equity Ownership. Shayan: Alexion Pharmaceuticals, Inc.: Employment, Equity Ownership. Hillmen: Gilead: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria; Celgene: Research Funding; Roche: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.