Abstract
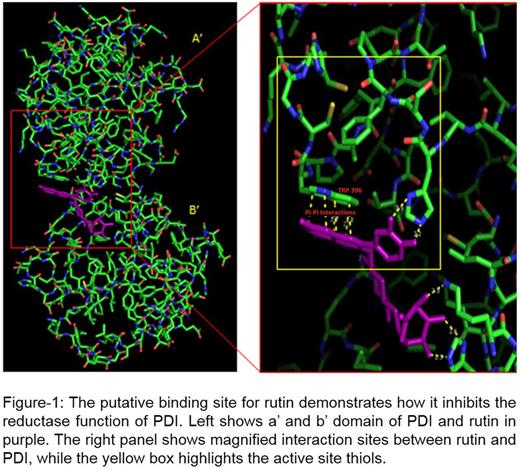
TGF-β1 is a very potent cytokine that, when activated, exerts both physiological and pathological functions, but the mechanism of activation remains unclear. Platelets contain 40-100-fold more TGF-β1 than other cell types and release TGF-β1 in an inactive latent complex comprised of mature TGF-β1 bound with latency-associated peptide and latent-TGF-β1 binding protein 1. We previously demonstrated that shear force activates latent TGF-β1 released from platelets in vitro, and that thiol-disulfide exchange contributes to this process. We also showed that protein disulfide isomerase (PDI) regulates shear-mediated TGF-β1 activation differentially, depending on whether PDI remains in its oxidized or reduced state. To test whether oxidation is required for latent TGF-β1 activation, we subjected platelet releasates to physiological shear/stirring for 2 hours in a closed chamber with room air or argon gas. We found ~10-fold increase in TGF-β1 activation compared to unsheared samples in room air, while the samples sheared under argon gas resulted in ~25% lower TGF-β1 activation (21 ± 1.0 % in room air vs. 16 ± 2.0 % of total TGF-β1 in argon gas, p=0.03). We also found that adding H2O2 to the platelet releasate resulted in increased shear-dependent TGF-β1 activation (active TGF-β1 was 16 ± 2.0 % in sheared samples and 22 ± 2.2 % of total TGF-β1 in sheared with H2O2 samples, p=0.03), indicating that oxidation during shear contributes to TGF-β1 activation. Since PDI can catalyze both oxidation and reduction of substrates, and because we previously showed that the reduced form of PDI inhibited shear-dependent TGF-β1 activation, we hypothesized that blocking the reduction function of PDI may increase shear-dependent TGF-β1 activation. To test this hypothesis, we assessed the effect of quercetin-3-rutinoside (rutin), a specific PDI inhibitor identified by high throughput screening, on shear-induced TGF-β1 activation. Rutin increased shear-induced TGF-β1 activation in a dose-dependent manner in that > 2.0 fold higher TGF-β1 activity was observed with 25 uM rutin, a concentration that blocked 55 to 80% of insulin reduction by PDI, confirming that rutin increases shear-dependent TGF-β1 activation by blocking the reduction function of PDI. We then assessed how rutin blocks PDI reduction function using PyMol modeling to identify the most likely interactions between rutin and PDI domains. After adjusting for the possible rotations of likely bond orientations, and considering minimum steric forces, the best model identified strong pi-pi interactions between Trp396 of the b' domain of PDI with the quercetin moiety of rutin (Figure-1). This interaction may stabilize the active site sulfhydryls of PDI so they maintain an inactive conformation, which buries the active site sulfhydryls, causing a loss in their solvent accessibility and thus inhibiting PDI's reductase function and increasing TGF-β1 activation. We conclude that thiol isomerases differentially regulate shear-dependent TGF-β1 activation. Thus, thiol-disulfide exchange regulator, such as PDI may be a potential target for suppressing or enhancing TGF-β1 activation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.