Abstract
Objective: We hypothesized that we could create and deploy a clinician orchestrated pediatric customized; three-dimensional virtual reality (VR) platform for distraction during IV procedures during a comprehensive hemophilia care visit without lengthening procedure time. We additionally hypothesized that VR would be favourably viewed by patients, caregivers,and clinical orchestrators.
Methods: We designed a single-center, randomized pilot study to assess the feasibility and usability of a customized pediatric VR environment during IV procedures in a pediatric hemophilia clinic.Participants were children >6 years to <19 years with hemophilia A or B followed in the Nationwide Children's Hospital Comprehensive Hemophilia Clinic. After informed consent, patients were block randomized 2:1 via computer generated randomization prior to a clinically indicated IV procedure to VR versus standard of care (SOC) distraction and age cohorts were maintained so that 12 patients were in age cohort 1 and 12 were in age cohort 2 (age cohort 1=>6y-<13y; and age group 2=>13y-<19y). Each subject, 1 caregiver and 1 hemophilia nurse orchestrator assessed the distraction method using an appropriately anchored initial Visual Analog/FACES scale prior to the IV procedure. Each subject, one caregiver and a hemophilia nurse orchestrator assessed the distraction method using a Final Visual Analog/FACES scale at the completion of the IV procedure.A score of 0 represented the most positive response and 100 represented the most negative response.Questions targeted usability, engagement, impact on procedural anxiety, impact on procedural pain, likability of the distraction technique, and procedural related nervousness. Additional data collected included type and severity of hemophilia, sex, historical use of prophylactic or on-demand therapy, and use of eyeglasses. To compare the length of procedure time between the two groups, the Wilcoxon Rank Sum test was used. The length of procedure times were summarized by presenting median and range.Kruskal-Wallis tests were utilized to compare VAS/FACES scores between raters.
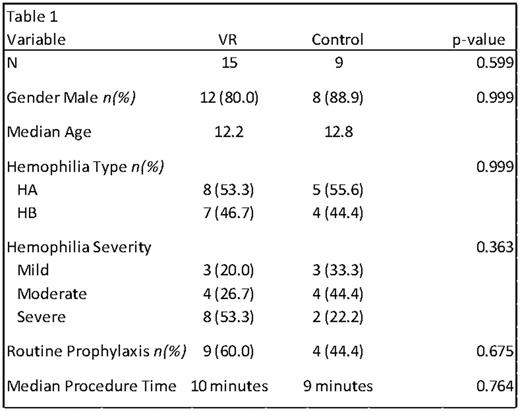
Results: We enrolled 25 children. One patient's data was censored due to inability to fit the VR headset over his glasses. In the remaining 24 participants there was no significant difference in group allocation between sex, age, type of hemophilia, hemophilia severity or use of prophylaxis (Table 1). In the 24 remaining patients, the median VR procedure time was 10 minutes and the median SOC time was 9 minutes (p=0.6085). There was no difference in scores between groups in terms of nervousness prior to IV, or impact of distraction technique on level of engagement, impact on pain, anxiety or likability. In the VR cohort, the median scores for ease of use and desire to use VR for future procedures was exceedingly positive for patients (7,12) nurses (2,3) and parents (9,8).
Conclusion: We demonstrated that a nurse orchestrated pediatric customized; three-dimensional VR environment during IV procedures could be integrated into a clinical setting when children with hemophilia underwent routine IV interventions during a comprehensive care visit without adversely disrupting clinic flow. Patients, nurses and caregivers expressed interest in using VR for future procedures. Future directions include validation of our findings in regard to clinical integration in multi-center hemophilia trials as well as other patient populations. Additional study regarding the impact of this platform on procedural pain and anxiety is warranted.
Dunn: Bayer: Consultancy, Other: Unrestricted educational grant; CSL Behring: Consultancy, Other: Unrestricted educational grant; Biogen: Other: Unrestricted educational grant, Research Funding; Kedrion: Other: Unrestricted educational grant; NovoNordisk: Other: Unrestricted educational grant; Octapharma: Other: Unrestricted educational grant; Shire: Consultancy, Other: Unrestricted educational grant, Research Funding; World Federation of Hemophilia USA: Membership on an entity's Board of Directors or advisory committees; Alnylam: Other: Unrestricted educational grant. Biega: Shire: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.