Abstract
BACKGROUND: Despite excellent outcomes in pediatric ALL, patients who relapse or become refractory to frontline therapies do poorly (Gaynon et al, BJH 2005). In pediatric AML, five-year overall survival remains around 60% among children and adolescents overall with similarly poor prognosis for relapsed or refractory AML (Wells et al, JCO 2003). Novel combinations with improved complete response rates and long term outcomes are required for these high risk patients. Mitoxantrone and clofarabine as single agents, have proven effective in children with relapsed/refractory ALL and AML and offer possible synergistic activity in vivo with less drug resistance. (Wells et al, JCO 2003; Jeha et al, JCO 2009; Chow et al, Leukemia & Lymphoma 2000). We have previously reported our Phase I results using this combination and have shown excellent early results. The MTD of this combination was demonstrated to be Clofarabine 35mg/m2 x 5 days and Mitoxantrone 12mg/m2 x 4 days. Here, we present data on the first 14 patients to be treated at this recommended Phase 2 dose level (RP2D).
OBJECTIVES: To determine the overall response rate of clofarabine in combination with mitoxantrone as reinduction therapy in children, adolescents and young adults with refractory/relapsed acute leukemia. To determine the percent of minimal residual disease (MRD) following reinduction with mitoxantrone and clofarabine reinduction therapy. To further characterize the biology of childhood, adolescent and young adult refractory/relapsed acute leukemia.
DESIGN: Prospective, multi-center, treatment-based, non-randomized, open label, uncontrolled, single group assignment, safety and efficacy study (NCT01842672). Patients 0-30.99yr old with ALL or AML with relapse OR primary induction failure were given 1 to 3 cycles of clofarabine (established MTD 35mg/m2/day) Day 1-5, in combination with mitoxantrone 12mg/m2/day on Day 3-6 (MITCL). Dexrazoxane was given prior to Mitoxantrone. CNS prophylaxis was achieved with intrathecal liposomal cytarabine. Patients were allowed subsequent cycles pending response and anthracycline exposure. The majority of patients were planned to undergo allogeneic hematopoietic stem cell transplantation upon achieving CR/MRD negative remission. MRD was defined by flow cytometry (≥ 0.01%)
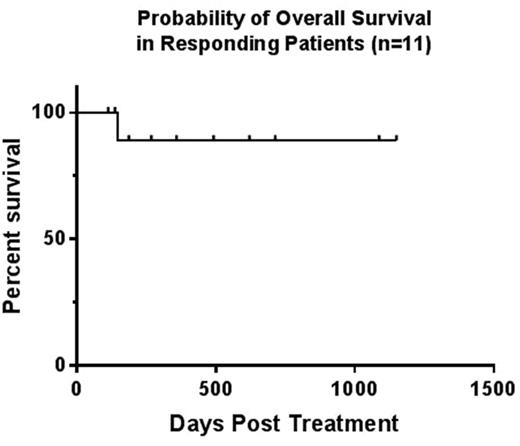
RESULTS: To date 14 patients have been enrolled at the established RP2D of clofarabine (six patients during Phase I, 8 patients during Phase II). Median Age is 6yrs (2-22yrs). Patient characteristics include 9 ALL (5=Induction Failure, 3=Relapse 1, 1=Relapse 2) and 5 AML (3=Induction Failure, 2=Relapse 1). The reinduction regimen was well tolerated. There has been 1 patient with Grade IV prolonged myelosuppression following therapy. For the remaining patients, median time to neutrophil recovery was 24 days. Eleven of 14 (79%) patients achieved a CR after 1 cycle of therapy. Of these patients, 100% achieved MRD negativity (<0.1%). Three patients died of progressive disease after 1 cycle of therapy. All 11 patients achieving CR went on to receive an allogeneic HSCT with no documented transplant associated toxicities attributed to prior MITCL therapy. Baseline and post treatment echocardiograms were compared in patients who achieved CR. No patient had clinical evidence of deterioration of left ventricular systolic function prior to proceeding with AlloHSCT. One patient developed MRD (2.6%) at Day +270 post HSCT. The remaining 10 patients demonstrated continued complete remission with MRD negativity at a median follow up time of 321 days (range 113-1150 days). The overall and event free survival for patients who responded to therapy is 91%. (Figure 1)
CONCLUSION: The combination of clofarabine and mitoxantrone reinduction therapy for relapsed or refractory acute leukemia has been demonstrated to be safe and well tolerated in children, adolescents and young adults with poor risk acute leukemias. Hematopoietic recovery continues to be rapid and complete. Data from the first 14 patients enrolled at the RP2D of this combination is encouraging with 79% MRD negative CR rate in leukemic patients allowing patients to safely proceed to AlloHSCT. Longer follow up and a larger cohort is planned and ongoing.
Oesterheld: Shire Pharmaceuticals: Speakers Bureau. Cairo: Jazz Pharmaceuticals: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.