Abstract
Therapeutic strategies in patients with acute myeloid leukemia (AML) have remained relatively unchanged over the last 30 years. AML is only cured in 35-40% of patients <60 years of age and in 5-15% of patients >60 years of age. Elderly patients who are unfit to receive intensive salvage regimens can only be offered low-intensity therapy or best supportive care. In children, AML accounts for 20% of leukemias. Although intensive multi-agent chemotherapy in conjunction with improved supportive care has increased survival rates to 60-70%, 30-40% of children with AML relapse and only one-third of them will survive to adulthood. The development and delivery of new therapeutic strategies for high-risk AML, including immunotherapy, therefore remains a priority.
Tumor phenotypes are dictated not only by the neoplastic cell component, but also by the tumor microenvironment (TME), which includes immune and inflammatory cells and mediators. We have previously shown that the treatment of primary AML cells with interferon (IFN)-γ in vitro induces functional indoleamine 2,3-dioxygenase-1 (IDO1), a molecule with potent immunosuppressive properties, in 50% of unselected cases ('IFN-γ responders'). With a median follow-up of 8 years, these IFN-γ responders experienced a significantly shorter event-free survival and overall survival.
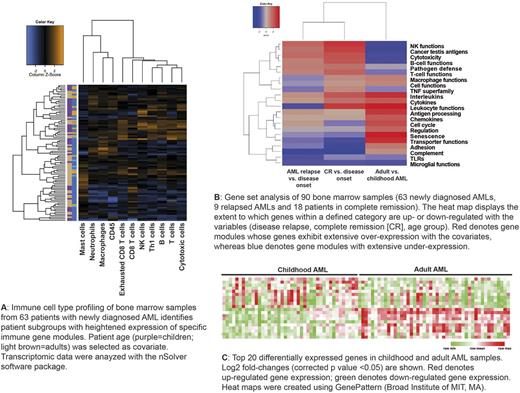
This multi-institutional study was undertaken to collect comprehensive immune profiles across the biological heterogeneity of childhood and adult AML, with the aim to implement new molecularly targeted agents for patients with specific immunologic subtypes of AML. We employed the nCounter™ system (NanoString Technologies, Seattle, USA), a medium-throughput, fully automated digital platform, to characterize bone marrow (BM) specimens from 70 patients with non-promyelocytic AML (42 children and 28 adults). Ninety BM samples (63 from patients with de novo AML, 18 from patients with AML in complete remission [CR] and 9 from patients with relapsed AML) were analyzed on the nCounter® FLEX platform, using the RNA Pan-Cancer Immune Profiling Panel. This panel detects the expression of genes for 109 cell surface markers on 24 immune cell types, 30 cancer testis antigens, and >500 immune response genes. Transcriptomic data were normalized using the nSolver™ software package (alpha version 4.0). Patient age and status (disease onset, achievement of CR and disease relapse) were selected as covariates.
Hierarchical clustering identified patient subgroups with heightened expression of CD8 T-cell, T helper type 1 (Th1)-cell, B-cell and cytotoxicity-related genes, including genes related to natural killer (NK)-cell function, in the leukemia microenvironment ("immune enriched" patients) (Fig. 1A). Interestingly, a select group of patients over-expressed genes associated with monocyte/macrophage, neutrophil and mast cell functions, with low expression levels of T-cell and B-cell genes. AML patients with 'immune enriched' gene profiles also expressed CD8A , IFNG , FOXP3 , the T-cell chemoattractants CXCL9 , CXCL10, and inhibitory molecules including IDO1 and the immune checkpoints LAG3 , CTLA4 and PDL1 . This immune landscape is consistent with a 'T-cell inflamed' phenotype that might underpin the establishment of adaptive immune resistance and immune escape. Gene set analyses identified differences in immune gene expression levels between childhood and adult AMLs, as well as patients with newly diagnosed AML, relapsed AML or AML in CR (Fig. 1B-C). In particular, genes highly expressed in adult AMLs compared with childhood disease included chemokine, dendritic cell and macrophage genes . In contrast, immune genes down-regulated in adult compared with childhood AMLs included integrin family members, cytokine receptors, T-cell receptor complex components ( CD3EAP ) and lipocalin-2 ( LCN2 ), a neutrophil gelatinase-associated molecule implicated in suppression of tumor invasiveness and metastasis.
In conclusion, our analysis has identified heterogeneous immunological profiles in children and adults with AML at different disease stages. From a clinical standpoint, 'immune enriched' AMLs might be amenable to immunotherapy approaches tailored to the BM microenvironment, including blockade of co-inhibitory molecules and/or small-molecule IDO1 inhibitors.
Grant support: Roger Counter Foundation, UK; Qatar National Research Fund (#NPRP8-2297-3-494).
Warren: NanoString Technologies: Employment, Equity Ownership. Hood: NanoString Technologies: Employment, Equity Ownership. Danaher: NanoString Technologies: Employment, Equity Ownership. Cesano: NanoString Technologies: Employment, Equity Ownership. Beechem: NanoString Technologies: Employment, Equity Ownership. Tasian: Gilead Sciences, Inc.: Research Funding; Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Rutella: NanoString Technologies, Seattle, USA: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.