Abstract
Introduction: Constitutive activation of the PI3Kδ pathway is associated with increased malignant B-cell proliferation and survival. INCB050465 is a highly selective and highly potent next-generation PI3Kδ inhibitor (≥19,000-fold more selective for PI3Kδ versus other isoforms), designed to eliminate hepatotoxicity associated with first-generation PI3Kδ inhibitors. Here we report safety and efficacy results from a phase 1/2 study of patients with relapsed or refractory B-cell malignancies treated with INCB050465 monotherapy (NCT02018861).
Methods: Adult patients were eligible for study participation if they had relapsed or refractory lymphoid B-cell malignancies (excluding Burkitt lymphoma and precursor B-cell lymphoblastic leukemia/lymphoma), Eastern Cooperative Oncology Group performance status score ≤2 (≤1 during dose escalation), normal hepatic and renal function, ≥1 prior treatment regimen, and no autologous hematopoietic stem-cell transplant (HSCT) within 3 months or allogeneic HSCT within 6 months of screening. The protocol was initiated with a single-patient cohort, treated with oral INCB050465 5 mg once daily (QD). Subsequent cohorts used a 3+3 design and evaluated doses of 10-45 mg QD. Based on pharmacokinetics/pharmacodynamics, the 20 and 30 mg QD cohorts were expanded. In November 2016, the treatment schedule was modified to introduce once-weekly (QW) dosing after week 9, based on emerging PK, safety, and efficacy data. Treatment responses were assessed every 9 weeks by the Lugano Classification, International Working Group on Chronic Lymphocytic Leukemia (CLL) criteria, or the 6th International Workshop on Waldenstrom Macroglobulinemia (WM) criteria as appropriate. Mandatory Pneumocystis jirovecii pneumonia (PJP) prophylaxis was implemented in May 2017.
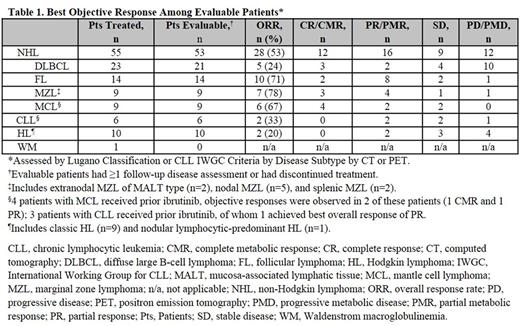
Results: As of the data cutoff (May 20, 2017), 72 patients were treated (median age, 66 [range, 30-89] years). Baseline tumor subtypes treated included NHL (diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, marginal zone lymphoma), Hodgkin lymphoma, CLL, and WM (Table 1). Sixty percent of patients received ≥3 prior systemic regimens. Median duration of exposure was 16.4 (range, 1-85.7) weeks; no dose-limiting toxicities occurred. Overall, 76% of patients discontinued therapy, most commonly due to disease progression (40%) and treatment-emergent adverse events (TEAEs; 18%). Dose interruption occurred in 39% of patients and dose reduction in 6% of patients due to TEAEs.
Most common nonhematologic TEAEs (all grade [Gr]; Gr ≥3) were nausea (36%; 0%), diarrhea (33%; 6%), fatigue (29%; 0%), vomiting (24%; 0%), and cough (22%; 0%). Gr ≥3 hematologic TEAEs were neutropenia (19%), lymphopenia (18%), thrombocytopenia (10%), leukopenia (8%), and anemia (6%). Serious TEAEs were reported in 38% of patients, most frequently diarrhea (n=5), pyrexia (n=5), hypotension (n=3), and colitis (n=3). One patient had grade 3 pneumonitis; none had PJP or grade >1 elevated transaminase while on study treatment.
Of note, among 16 patients with NHL who received QW dosing, none discontinued treatment because of a TEAE while on the QW schedule (median treatment duration on the QW schedule, 16.2 [range, 0.7-28.7] weeks).
Of 72 patients treated, 69 (96%) were evaluable for response (Table 1); objective responses occurred at all doses, except 5 mg QD. Among patients with NHL, 93% (26/28) of the objective responses were observed at 9 weeks. All 16 patients receiving QW dosing had a response/stable disease during QD dosing; 9 patients remained on the QW schedule at the data cutoff date.
Conclusion: In patients with relapsed or refractory B-cell malignancies, INCB050465 demonstrated manageable toxicities with no clinically meaningful transaminitis or PJP. Objective response rates were generally high and 93% of patients with NHL who responded did so at the first assessment. The QW schedule appears to improve tolerability and prolong time on study treatment in NHL subtypes. Phase 1 and 2 studies are underway to investigate this and other dosing regimens in patients with NHL. Updated data will be presented.
Forero-Torres: TRACON Pharma (Inst): Research Funding; Pfizer: Research Funding; Oncothyreon: Research Funding; Syndax (Inst): Research Funding; Novartis: Research Funding; Daiichi Sankyo: Research Funding; Immunomedics: Research Funding; Gilead Sciences (Inst): Research Funding; Genentech/Roche: Research Funding; Seattle Genetics: Research Funding, Speakers Bureau. Ramchandren: Curis: Consultancy; Gilead Sciences: Consultancy; Seattle Genetics: Consultancy; Janssen: Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding. Yacoub: Pfizer: Consultancy; Novartis: Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Sandoz: Consultancy; Seattle Genetics: Consultancy, Speakers Bureau; CTI: Consultancy. Wertheim: Millennium: Speakers Bureau; Lilly: Speakers Bureau; Celgene: Speakers Bureau; AstraZeneca: Speakers Bureau. Edenfield: Novartis: Speakers Bureau; Astellas Medivation: Speakers Bureau. Caimi: Incyte: Equity Ownership; Abbvie: Equity Ownership; Seattle Genetics: Equity Ownership; Celgene: Speakers Bureau. Gutierrez: MedImmune: Research Funding; TG Therapeutics: Research Funding; Rexahn Pharmaceuticals: Research Funding; Novartis: Research Funding; Gilead Sciences: Research Funding; Lilly: Consultancy, Research Funding; Karyopharm Therapeutics: Consultancy, Research Funding; Bayer: Consultancy; COTA: Equity Ownership; Merck: Research Funding, Speakers Bureau; Acceleron Pharma: Research Funding; Incyte: Research Funding; Daiichi Sankyo: Research Funding; Bristol-Myers Squibb: Research Funding, Speakers Bureau; Loxo: Research Funding; EMD Serono: Research Funding; Esanex: Research Funding; Foundation One Inc: Speakers Bureau; Abbvie: Research Funding. Akard: Gilead Sciences: Speakers Bureau; Millennium: Speakers Bureau; Incyte: Research Funding; Celgene: Speakers Bureau; Cellerant: Research Funding; Bristol-Myers Squibb: Speakers Bureau; Teva: Research Funding, Speakers Bureau; Astellas Medivation: Research Funding; Pfizer: Research Funding; Unum Therapeutics: Research Funding; Novartis: Consultancy, Speakers Bureau. Escobar: TOPA: Employment; Seattle Genetics: Speakers Bureau; VIMA: Membership on an entity's Board of Directors or advisory committees. Persky: Genentech: Consultancy; MorphoSys: Other: Independent Data Monitoring Committee member ; Verastem: Consultancy; Spectrum Pharmaceuticals: Research Funding. Iyer: Takeda: Research Funding; Genentech: Research Funding. DeMarini: Incyte: Employment, Equity Ownership. Zhou: Incyte: Employment, Equity Ownership. Yeleswaram: Incyte: Employment, Equity Ownership, Other: Travel/Expenses. Phillips: KITE: Consultancy; Seattle Genetics: Consultancy; Incyte: Other: Travel/Expenses; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.