Abstract
Introduction:
Patients (pts) diagnosed with MYC -rearranged non-Burkitt aggressive B cell lymphoma (MYC-R), including those with double hit lymphoma (DHL), are at high risk for developing relapsed/refractory (R/R) disease, even if treated with intensive immunochemotherapy (IIC). While a randomized prospective study of therapy intensification in diffuse large B cell lymphoma (DLBCL) pts with positive (+) interim PET-CT scan (iPET) did not result in a survival advantage (Blood 2016 128:1857), it may be relevant to explore alternation of therapy in iPET+ MYC-R pts, as a lack of substantial initial response to chemotherapy in a disease characterized by MYC -driven rapid cellular proliferation may predict for subsequent chemoresistance and treatment failure. Here we analyze outcomes for MYC-R pts based upon iPET status as well as baseline clinicopathologic and treatment characteristics.
Methods:
MYC-R pts were defined as those diagnosed with DLBCL or B cell lymphoma unclassifiable/high grade B cell histologic classification (transformed indolent lymphoma allowed if no prior cytotoxic therapy received) and ≥10% of cells within paraffin embedded tissue (≥1% for fresh tissue) demonstrating rearrangement or translocation of the MYC /8q24 locus by fluorescence in situ hybridization or metaphase karyotype. DHL was defined as MYC-R with concurrent rearrangement of BCL2 and/or BCL6 . Double expressor lymphoma (DEL) was defined as positivity by immunohistochemical staining (IHC) for MYC and BCL2 using cutoffs of 40% and 50%, respectively. All cytogenetic analyses and acquisition/interpretation of PET-CT images were performed at the University of Pennsylvania. Scoring of IHC on available tissue specimens was performed by an expert hematopathologist (RLS). Pts received front-line therapy with either R-CHOP or IIC regimens consisting of R-EPOCH or R-hyperCVAD at the discretion of the treating physician. iPET was performed after at least 2 cycles but prior to completion of 50% of front-line therapy, and iPET + was defined as Deauville score ≥4 and iPET negative(-) ≤3. No pt changed treatment regimen based upon iPET result. Response assessment was performed as per the Lugano Classification. Progression free survival (PFS) was defined as the interval between diagnosis and disease progression or last follow-up in remission and overall survival (OS) between diagnosis and death or last follow-up. Pts were diagnosed from 2010-2016 and treated at the University of Pennsylvania. Data were censored on 7/1/2017.
Results:
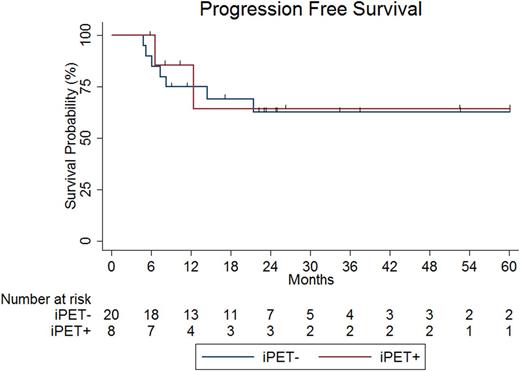
Twenty-eight pts were included in this analysis. Baseline clinicopathologic characteristics were as follows: 54% female, 39% age >60, 86% LDH >normal, 75% stage III-IV, 18% ECOG performance status >1, 71% extranodal disease, 61% IPI score ≥3, 21% transformed indolent lymphoma, 54% Ki67 ≥90%, 79% germinal center cell of origin by Hans algorithm, 50% MYC IHC ≥40%, 29% DEL, 50% DHL. Characteristics of treatment received included 71% IIC, 64% CNS prophylaxis, 36% consolidative radiation and consolidative autologous stem cell transplant in 1 pt. Eight (29%) pts were iPET+ and 20 (71%) pts iPET-. DHL occurred more frequently in iPET+ as compared with iPET- pts (88% vs. 35%, p=0.04); no other baseline clinicopathologic or treatment characteristic differed significantly between iPET+ and iPET- pts. With a median length of follow-up of 24.6 (range 5.7-98.7) months (mo), PFS at 24 mo (PFS24) and OS at 24 mo (OS24) were 64% and 73%, respectively. Neither PFS24 (64% vs. 63%, p=0.82, Figure) nor OS24 (100% vs. 67%, p=0.20) differed significantly between iPET+ and iPET- pts. For pts treated with IIC (n=20), PFS24 was 55% and OS24 68% and did not differ significantly in iPET+ as compared with iPET- pts. Univariate analysis demonstrated that no baseline clinicopathologic or treatment characteristic was predictive of PFS24 or OS24. For pts with DEL, PFS24 and OS24 were 49% and 63%, and for pts with DHL, 67% and 79%. The positive and negative predictive values of iPET for end-of-treatment PET-CT were 75% and 85%, respectively.
Conclusions:
PFS24 was 64% in this cohort of MYC-R pts largely treated with IIC and did not differ significantly based upon iPET result. If strategies to reduce the development of R/R disease, such as incorporation of novel therapeutics into front-line IC and/or consolidation/maintenance regimens, are to be investigated in MYC-R pts, they should not be limited to those who are iPET+.
Dwivedy Nasta: Immunogen: Research Funding; Takeda: Research Funding; Incyte: Research Funding. Svoboda: Celgene: Research Funding; Pharmacyclics: Research Funding; Merck: Research Funding; BMS: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Kite: Consultancy. Schuster: Seattle Genetics: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Nordic Nanovector: Consultancy; Gilead: Consultancy, Research Funding; Merck: Research Funding; Novartis: Consultancy, Research Funding. Mato: TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Consultancy; Pharmacyclics: Research Funding; Acerta: Research Funding; AstraZeneca: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; DTRM: Research Funding; Portola: Research Funding; Regeneron: Research Funding; AbbVie: Consultancy, Research Funding; Janssen: Consultancy; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees. Morrissette: Novartis: Consultancy. Landsburg: Takeda: Research Funding; Curis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.