Abstract
Background: MDS comprise a heterogeneous sort of disorders caused by cytogenetic changes, gene mutations, or both, responsible for altered multilineage morphological patterns that lead to a varying grade of peripheral blood cytopaenias and an increased risk of transformation to acute myeloid leukemia. Thrombocytopaenia is observed in aproximately 35% of MDS patients. Despite it is accepted that haemorrhagic events are more likely at platelet counts below 10,000/μl, in the case of MDS patients, whose platelets had a diminished ability to be activated, bleeding events are lower than expected. So, hemostasis in MDS patients is a complex issue where mechanisms additional to platelet count/function seem to be involved.
Aim: This work aimed to identify procoagulant mechanisms in non-bleeders MDS patients that might protect them from bleeding.
Methods: This is a prospective, observational and transversal study. Eighty four non-bleeders MDS patients (44% female, mean age of 77±25 years), were included. Sixty healthy subjects (45% female, mean age of 57±22 years) were recruited.
Human peripheral blood samples were collected in 3.8% sodium citrate. Blood cell counts were performed with a Coulter Ac.T Diff cell counter (Beckman Coulter, Madrid, Spain). Platelet rich plasma (PRP) and platelet free plasma (PFP) were obtained by centrifugation.
Surface exposure of phosphatidylserine (PS) and active caspase-3, -8 or -9 were assessed by flow cytometry measuring the binding of FITC-labeled Annexin V (BD Pharmingen) and with a specific kit for measuring these caspases (Millipore, Madrid, Spain).
Prothrombinase complex binding to platelets was determined in quiescent washed platelets (1x108/mL) incubated with factor (F) Xa (5 nmol/L) and FVa (5nmol/L) obtained from Haemtech Inc (Cellsystems, Germany). Following fixation to cross-link platelet-bound FVa and FXa, specific or control monoclonal antibodies ( 0.1 μmol/L each, Haemtech Inc, Germany) were added. All samples were analized by flow cytometry.
PS-associated and tissue factor (TF)-associated procoagulant activity of microparticles (MPs) was determined with the ZYMUPHEN MP-Activity kits (HYPHEN BioMed, Neuvillesur Oise, France).
The identification of the MP's cellular origin was determined by flow cytometry, labeling MPs with Annexin-V-FITC and the following specific mAb conjugated with phycoerythrin: anti-CD41 mAb for platelets (Biocytex, Marseille, France); and anti-CD14 mAb for monocytes, anti-CD144 mAb for endothelial cells, anti-CD235 mAb for red cells and anti-CD45 mAb for leukocytes, all from BD Biosciences.
The statistical analysis of the experimental data was using SPSS 9.0 software (SPSS Inc., Chicago, Illinois, USA).
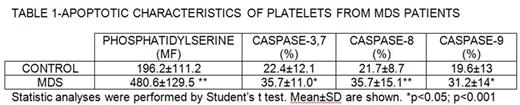
Results: Platelets from patients with MDS presented higher levels of activated caspases-3,7, -8 and -9 and this situation was accompanied by a higher PS platelet surface exposure (TABLE 1)
We evaluated if increased PS exposure was functional for anchoring prothrombinase complex. Binding of FVa and of FXa on platelet membrane surface was higher in quiescent platelets from MDS patients [FVa (Mean fluorescence), control: 49.7±8.5, MDS patients: 73.9±23.2, p<0.01; FXa (Mean fluorescence), control: 46.4±17.5 , MDS patients: 77.6±12.0, p<0.05)].
A significantly higher procoagulant capacity of MPs associated either to PS (control: 8.0±2.5 nanomol/L; MDS: 17.8±3.5 nanomol/L, p<0.01) or TF (control: 0.4±2.5 nanomol/L; MDS: 7.5±2.5 nanomol/L, p<0.001) were observed in the MDS group.
To determine the cellular source of MPs, flow cytometry analyses were performed and a significant increase was observed in the amount of monocyte- (events, control: 94±30, MDS patients: 220±29, p<0.05)and red cell-derived MPs (events, control: 142±55, MDS patients: 252±23, p<0.01) in the samples from the patients with MDS. In patients with MDS, TF-MP-associated thrombogenic ability correlated with monocyte percentage (Spearman r=0.5019, p<0.01).
Conclusion: MDS patients, even those with normal platelet count, have a reduced platelet function with enhanced apoptosis but do not bleed in many cases. This fact might be due to the increase in tissue factor-containing microparticles and in the enhanced binding of prothrombinase complex to apoptotic platelets which, in turn, augmented procoagulant capacity of these patients.
Work supported by grant from FIS-FEDER PI15/01457. NB holds a Miguel Servet II (FIS-FEDER CP14/00024).
Jimenez-Yuste: Roche: Consultancy; Novo Nordisk: Consultancy, Honoraria, Research Funding. Álvarez-Roman: Shire: Consultancy; Novo Nordisk: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.