Abstract
Background:
Chronic myelomonocytic leukemia (CMML) is an aggressive myeloid neoplasm with overlapping myeloproliferative and myelodysplastic features. Extramedullary manifestations (EMM) include splenomegaly, hepatomegaly, lymphadenopathy, leukemia cutis, gingival infiltrates, central nervous system (CNS) and testicular involvement and myeloid sarcomas. We carried out this study to i) define clinical and laboratory correlates of EMM in CMML, ii) to assess the prognostic impact of EMM, and iii) to document survival outcomes.
Methods:
Four hundred and fifty-two patients with WHO-defined CMML were included in the study. All morphological and cytogenetic assessments at baseline were performed at the Mayo Clinic, MN. Bone marrow (BM) DNA at diagnosis was available in 229 (50%) patients and was subjected to a 29 gene panel next generation sequencing analysis. EMM for the purpose of this study were defined as: palpable splenomegaly, palpable hepatomegaly, palpable lymphadenopathy, clinical/radiological or biopsy proven leukemia cutis, gingival infiltrates, testicular involvement, CNS involvement, and myeloid sarcomas. EMM were included if present either at the time of CMML diagnosis, or within six months of stable disease following diagnosis. Standard statistical tests and measures were utilized.
Results:
Three hundred and seventy-three (82%) patients had an exam documented at the time of diagnosis, or within six months of diagnosis, and were included in the analysis. The median age was 69 years (range, 18-95), 66% were male and 176 (47%) had a "proliferative CMML phenotype."
1. Clinical correlates:
One hundred and nineteen (32%) patients had at least one EMM at diagnosis. This included; palpable splenomegaly 91 (76%), hepatomegaly 48 (40%), lymphadenopathy 19 (15%), leukemia cutis 7 (6%), gingival infiltrates 4 (3%), myeloid sarcomas 2 (2%), and 1 each with testicular and CNS involvement (1%). Thirty two (27%) had non hepatosplenic EMM, 102 (86%) had palpable hepato and/or splenomegaly, whereas 54 (45%) and 11 (9%) had isolated palpable splenomegaly and hepatomegaly. In comparison to CMML patients without EMM, those with, were more likely to be younger (p <0.0001), have a higher leukocyte count (WBC, p <0.0001), higher absolute neutrophil count (ANC, p <0.0001), higher absolute monocyte count (AMC, p <0.0001), have circulating immature myeloid cells (IMC, p <0.0001), have mutations involving ASXL1 (p 0.03), CBL (p 0.04), and CSF3R (p 0.02), lack SF3B1 (p 0.009) and ZRSR2 (p 0.03) mutations and have a higher risk stratification by the Mayo Molecular Model (MMM, p<0.0001) and the GFM model (p=0.004).
2. Prognostic impact:
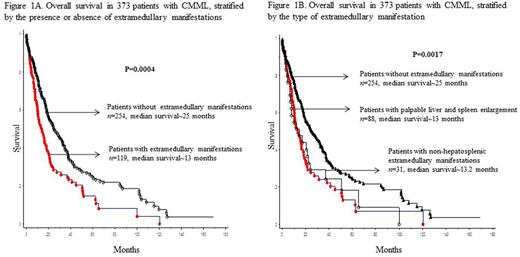
At last follow up (median 14 months), 249 (67%) deaths and 69 (18%) leukemic transformations were documented. Median survival (OS) was 13 months for patients with non hepatosplenic EMM, 13.2 months for those with palpable hepatosplenomegaly, and 25 months for those without EMM (Figure 1). On univariate analysis, survival was adversely affected by, male sex (p 0.03), low hemoglobin (p <0.0001), high WBC (p 0.0005), high AMC (p <0.0001), presence of IMC (p 0.002), circulating blast % (p <0.0001), high LDH (p 0.0004), presence of EMM (p <0.0001), palpable hepatosplenomegaly (p=0.0008), leukemia cutis (p=0.02), proliferative CMML phenotype (p <0.0001), adverse cytogenetics (p 0.003), absence of TET2 (p 0.0004) and the presence of DNMT3A (p 0.002), IDH1 (p 0.013), ASXL1 (p 0.03), and Tp53 (p 0.014) mutations. Non-hepatosplenic EMM, palpable lymphadenopathy, CNS, testicular involvement and the presence of myeloid sarcomas did not impact OS. On multivariate analysis, only male sex (p= 0.009, HR 1.6, 95% CI 1.1-2.3), low hemoglobin (p <0.0001, HR 1.74, 95% CI 1.3-2.3), palpable hepatosplenomegaly (p 0.0005, HR 1.8, 95% CI 1.04-2.2), absence of TET2 mutations (p 0.023, HR 0.7, 95% CI 0.5-0.95), and the presence of DNMT3A mutations (p 0.013, HR 2.3, 95% CI 1.2-4.3) retained an independent negative prognostic impact. Palpable hepatosplenomegaly retained prognostic significance when assessed in the context of the MMM (p=0.03) and the GFM (p=0.04) models. EMM had no impact on leukemia-free survival.
Conclusion:
The current study demonstrates a prognostically-relevant frequent occurrence of hepatosplenomegaly in CMML; the study also provides the spectrum and frequency of extramedullary manifestations and correlations with mutations.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.