Abstract
Introduction: Ibrutinib is approved for all lines of therapy in CLL with a recommended starting dose at 420mg daily. Dose interruptions ≥ 8 days may negatively impact both progression free survival (PFS) and overall survival (OS) (Barr et al, Blood 2017). However, dose reduction did not appear to affect responses or survival (Timlin et al, ASCO 2015; Mato A et al, BJH 2016). We aimed to comprehensively study the impact of dose interruptions and ibrutinib starting dose (< 420 mg) on survival outcomes in front-line ibrutinib use.
Methods: We conducted a multicenter, retrospective cohort study of CLL patients treated with front-line ibrutinib. We recorded and categorized ibrutinib starting dose (reduced dose vs. 420 mg daily) and number of days required for dose interruptions (0-7 days vs. ≥ 8 days). Study endpoints included overall response rates, PFS and reasons for discontinuation. Comparisons of outcomes data were made using COX regression. All other comparisons were descriptive.
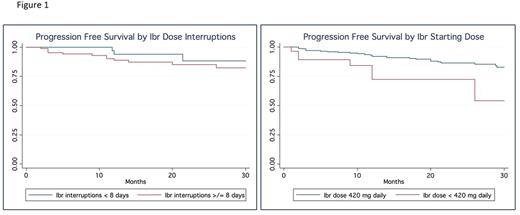
Results: We identified 391 patients treated with front-line ibrutinib. Of these, 30 (7.6%) initiated treatment at doses < 420 mg (47% 140 mg daily and 53% 280 mg daily). For the reduced dose cohort, median age was 76 years (range: 47-96) vs. 67 years (range: 36-96) in the standard dose cohort, 60% were males, and 83% Caucasians. Prognostic factors included: 27% del17p+ (vs. 30% in the standard dose cohort), 78% IGHV unmutated (vs. 66%), 21% complex karyotype (vs. 24%). In this cohort, the ORR was 85% (vs. 81% in the full dose cohort), but 12 month PFS was 71% (vs. 93% in the full dose). This inferior PFS was significant (HR 3.3, p=0.003; 95%CI: 1.57.0). Eighty-six (22%, 86/391) patients had a dose interruption of ≥ 8 days (median duration = 14 days). The 12 month PFS was not impacted with dose interruption (90% if ≥ 8 days vs. 96% if <8 days) (HR 1.48, CI .48-4.6 p=0.49). At the time of this analysis, 8 patients discontinued reduced dose ibrutinib. Reasons for discontinuation included: 4 patients for toxicity, 2 patients for CLL progression and 1 patient for Richter's transformation. Median time to discontinuation was 10.5 months for patients who initiated treatment on a reduced dose of ibrutinib. Figure 1 describes PFS stratified by dose interruptions (<8 days vs ≥8 days) and ibrutinib starting dose (<420 mg daily vs 420 mg daily).
Conclusions: While response rates appear to not be affected when ibrutinib is initiated at lower than 420 mg daily, patients starting at lower doses appear to have inferior PFS. Unlike in high-risk heavily pretreated patients, the PFS of patients receiving front-line ibrutinib was not adversely affect by dose interruptions of ≥ 8 days. To our knowledge, these data are the first to show a relationship between front-line ibrutinib dosing and outcomes. These data support dosing and dose interruption recommendations as guided by the ibrutinib FDA label.
Barr: Gilead: Consultancy; Novartis: Consultancy; Infinity: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Celgene: Consultancy; AbbVie: Consultancy, Research Funding; Seattle Genetics: Consultancy. Ujjani: Genentech: Consultancy; Abbvie: Research Funding, Speakers Bureau; Gilead: Consultancy; Pharmacyclics: Consultancy, Research Funding. Tam: Janssen Cilag: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding. Lansigan: Seattle Genetics: Consultancy; Spectrum Pharmaceuticals: Consultancy, Research Funding. Brander: Teva Pharmaceuticals, Genentech, AbbVie, Pharmacyclics: Consultancy; Genentech: Consultancy; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Shadman: TG Therapeutics: Research Funding; Celgene: Research Funding; Pharmacyclics: Other: advisory board, Research Funding; Emergent: Research Funding; AbbVie: Other: advisory board; Genentech: Consultancy, Research Funding; Gilead: Research Funding; PLEXXIKON: Research Funding; Acerta Pharma: Research Funding; Merck: Research Funding. Skarbnik: Seattle Genetics: Speakers Bureau; Genentech: Speakers Bureau; Abbvie: Other: Ad board, Speakers Bureau; Gilead: Speakers Bureau; Novartis: Speakers Bureau. Pagel: Pharmacyclics: Consultancy; Gilead: Consultancy. Cheson: Acerta, Pharmacyclics, Epizyme, Gilead, Roche, AbbVi: Other: Institution receives research support ; AbbVie, Roche-Genentech, Pharmacyclics, Acerta: Consultancy. Furman: Gilead: Consultancy; Pharmacyclics: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Genentech: Consultancy; Sunesis: Consultancy; TG Therapeutics: Consultancy; Verastem: Consultancy. Mato: Acerta: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; DTRM: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Regeneron: Research Funding; Portola: Research Funding; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Janssen: Consultancy; AstraZeneca: Consultancy; Kite: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.