Abstract
Introduction
A triplet combination including a proteasome inhibitor (PI) and an IMiD has shown significant efficacy in the setting of newly diagnosed multiple myeloma (NDMM). In addition, a role for maintenance therapy with the PI bortezomib has been suggested in non-head to head comparisons. However, in elderly patients especially non-hematological toxicity is a concern and an oral drug is preferred as maintenance therapy. Therefore, we investigated the efficacy and feasibility of an oral regimen including induction therapy with the PI ixazomib in combination with thalidomide, followed by a randomization between maintenance therapy with either ixazomib or placebo in elderly non-transplant eligible (nte) NDMM. We here report the efficacy and toxicity of the induction therapy of the first 120 patients, with subgroup analyses based on frailty and cytogenetic risk. The study is currently ongoing and results with longer follow up and the maintenance part will be reported at future meetings. This trial was registered at www.trialregister.nl as NTR4910.
Methods
In this prospective multicenter phase II trial nte-NDMM patients were treated with nine 28 day-cycles consisting of ixazomib 4mg (day 1, 8, 15), thalidomide 100 mg (day 1-28) and dexamethasone 40 mg (day 1, 8, 15, 22) followed by randomisation between either ixazomib or placebo (both day 1, 8, 15 every 28 days) until progression. Primary objectives were comparison of progression free survival (PFS) between maintenance therapy with ixazomib or placebo and to determine the overall response rate of induction therapy.
Frailty was assessed by a modification of the IMWG frailty index based on age, the Charlson Comorbidity Index (retrospectively retrieved from the list of comorbidities that were present at entry) and the WHO performance as a proxy for (instrumental) Activities of Daily Living (scoring WHO 0 as 0 points, WHO 1 as 1 point, and WHO 2-3 as 2 points).
High risk cytogenetics was defined as del17p, t(4;14) or t(14;16).
Results
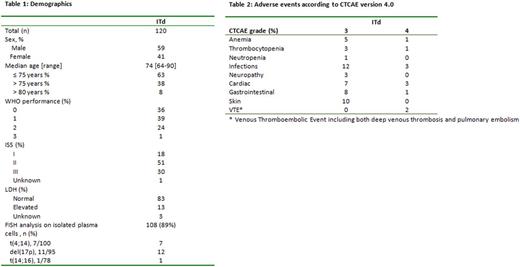
Patient characteristics are presented in table 1. The median follow up is 17.0 months (range 0.9-28.1 months). Following induction treatment overall response rate (ORR, i.e. ≥PR) was 81% (95% confidence interval (CI) 73-87%), 44% (95% CI 35-54%) of patients reached ≥ VGPR and 10% (95% CI 5-17%) ≥ CR. ORR was 85% in patients ≤75 years versus 73% in ≥76 years (p=0.15), while for ≥VGPR these were 45% vs 42% (p=0.85) and for ≥CR 13% vs 4% (p=0.21). In responding patients, the median time to response was 1.1 month.
Using the revised IMWG Frailty Index 47% of patients were frail, 28% unfit and 21% fit (4% unknown). Frailty status did not affect the efficacy of induction therapy with ITd with ORR 75%, ≥ VGPR 43% and ≥ CR 9% in frail versus 85%, 53% and 9% in unfit and 88%, 36% and 16% in fit patients (p=0.34, p=0.43 and p=0.62, respectively).
At the time of this analysis cytogenetic risk could be determined in 77/120 (64%) patients, with 23% of high risk patients. Response rates were comparable in high versus standard risk patients: ORR 14/18 (78%) versus 50/59 (85%), ≥ VGPR 7/18 (39%) versus 25/59 (42%) and ≥ CR 1/18 (6%) and 4/59 (7%) (p=0.49, p=1.0 and p=1.0).
Toxicity is presented in table 2. The incidence of severe neuropathy was low; 3% grade 3 and no grade 4. During induction 18/120 (15%) patients discontinued therapy due to toxicity; 6 neurotoxicity, 3 infection, 2 dermatological toxicity, 2 gastro-intestinal toxicity and 5 other toxicity. Discontinuation was comparable in patients ≥76 versus age ≤75, however none of the 10 patients > 80 years reached maintenance therapy, of whom 6/10 due to toxicity. Early mortality was higher in patients ≥76; 4/45 (9%) versus 1/75 (1%) in patients ≤75, mainly due to infections (4/5). The discontinuation rate due to toxicity was 1/25 (4%), 5/34 (15%) and 9/56 (16%)(p=0.34) in fit, unfit and frail patients respectively.
Conclusion
Induction treatment with ITd in NDMM results in high overall response rate of 81%, with 44% ≥ VGPR. Median time to response was 1.1 month. In frail and high risk cytogenetic patients induction therapy with ITd was equally effective with respect to response. Therapy was found to be feasible with limited toxicity and a low resulting discontinuation rate of 15%, although in patients >80 years toxicity was higher.
Zweegman: Janssen: Other: advisory board participation, Research Funding; Celgene: Other: advisory board participation, Research Funding; Amgen: Other: advisory board participation; Takeda: Other: advisory board participation, Research Funding. Waage: Celgene, Takeda: Honoraria. Sonneveld: Celgene, Amgen, Janssen, Karyopharm, Takeda: Consultancy, Honoraria, Research Funding; Celgene Corporation, Amgen, Janssen, Karyopharm, SkylineDx, PharmaMar: Consultancy; Celgene Corporation, Amgen, Janssen, Karyopharm, PharmaMar, SkylineDx: Honoraria. Abildgaard: Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.