Abstract
Background: The introduction of several novel agents including immunomodulators, proteasome inhibitors, and monocloncal antibodies has lead to an improvement in response rates and survival outcomes in patients with multiple myeloma. Despite these recent advances the majority of patients experiences disease relapse and eventually refractory disease. Given the availability of several therapy regimens to choose from, predictive biomarkers are needed to help guide treatment decisions.
Methods: We studied 358 patients with newly diagnosed (n = 85) or relapsed / refractory (n = 273) multiple myeloma who were treated on 20 prospective phase I/II and phase II clinical trial protocols between February 2004 and August 2016 at Mayo Clinic. All patients had measurable disease (M-spike ≥ 1 g/dL and involved free light chain ≥ 10 mg/dL). All patients had M-spike and free light chain measurements available before and after the first treatment cycle. Response to treatment was evaluated using the International Myeloma Working Group Uniform Response Criteria. The serum parameters of interest were the relative decrease in M-spike and absolute free light chain difference (ΔFLC). The Wilcoxon signed-rank test was used to compare the serum parameters before and after the first treatment cycle. Logistic regression models were used to assess the associations between the change in serum parameters after the first treatment cycle and best response during the same treatment course. Proportional hazards regression models were used to assess the associations between the change in serum parameters after the first treatment cycle and overall survival. P-values below 0.05 were considered statistically significant.
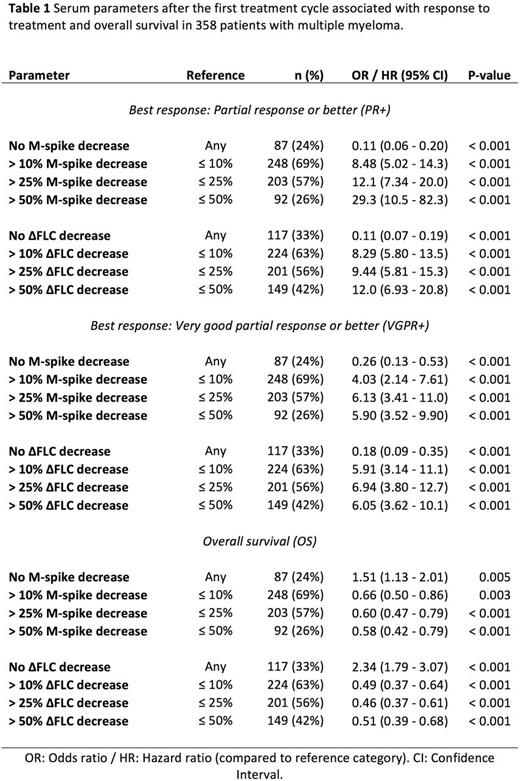
Results: The median age at diagnosis was 62 years (33 - 86), 208 patients (58%) were male. The five most common regimens were pomalidomide + dexamethasone, lenalidomide + cyclophosphamide + dexamethasone, single agent ixazomib, ixazomib + cyclophosphamide + dexamethasone, and lenalidomide + dexamethasone. After a median follow-up of 3.1 years (0.1 - 13.3) the median overall survival was 4.3 years (95% CI 3.9 - 4.9). The median baseline M-spike decreased from 2.1 g/dL (0.0 - 7.4) to 1.4 g/dL (0.0 - 7.0) after the first treatment cycle (p < 0.001). The median baseline ΔFLC decreased from 19.8 mg/dL (0.0 - 3499.9) to 8.4 mg/dL (0.0 - 6639.9) after the first treatment cycle (p < 0.001). The 87 patients (24%) who did not experience a decrease in M-spike after the first treatment cycle had decreased odds of responding (partial response or better) to treatment later on (OR 0.11, 95% CI 0.06 - 0.20, p < 0.001). The median survival in those with a decrease in M-spike was longer (4.7 years, 95% CI 4.0 - 5.3) compared to those without (2.9 years, 95% CI 1.7 - 4.0, p = 0.005). The 117 patients (33%) who did not experience a decrease in ΔFLC after the first treatment cycle had decreased odds of responding (partial response or better) to treatment later on (OR 0.11, 95% CI 0.07 - 0.19, p < 0.001). The median survival in those with a decrease in ΔFLC was longer (5.2 years, 95% CI 4.6 - 6.0) compared to those without (1.8 years, 95% CI 1.4 - 3.1, p < 0.001). Greater decreases in M-spike and ΔFLC after the first treatment cycle were associated with increased odds of responding to treatment and better overall survival. The serum parameters associated with response to treatment and overall survival are summarized in Table 1.
Conclusions: Patients with multiple myeloma treated on clinical trial protocols were unlikely to respond to treatment if they did not experience a decrease in M-spike and ΔFLC after the first treatment cycle. Decreases in M-spike and ΔFLC were predictive of response to treatment and were of prognostic significance in this population. These readily available biomarkers may help to guide treatment decisions in patients with multiple myeloma.
Gertz: Millennium: Consultancy, Honoraria; Celgene, Novartis, Smith-Kline, Prothena, Ionis, Amgen: Honoraria. Dispenzieri: Celgene, Millenium, Pfizer, Janssen: Research Funding. Russell: Imanis Life Sciences: Equity Ownership; Vyriad: Equity Ownership. Kapoor: Takeda, Celgene and Amgen: Research Funding. Kumar: Skyline: Honoraria; Celgene, Millennium, BMS, Onyx, Janssen, Noxxon, AbbVie, Amgen, Merck, Oncopeptides, Skyline Diagnostics, Takeda: Consultancy; Celgene, Millennium/Takeda, Onyx, AbbVie, Janssen, Sanofi, Novartis, Amgen, Genentech, Merck, Oncopeptides, Roche, Skyline Diagnostics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.