Abstract
INTRODUCTION: Autologous haematopoietic stem cell transplant(HSCT) with anti-thymocyte globulin (ATG) based conditioning is currently being explored for its efficacy and safety in the treatment of Multiple Sclerosis (MS). Despite increasing experience gained, the procedure still carries significant risk of morbidity. Epstein Barr Virus (EBV) infection and its role in pathogenesis of MS remain undetermined. EBV reactivation and post-transplant lymphoproliferative disorders (PTLD) are well described in allogeneic HSCT and uncommon with auto HSCT for other malignant conditions. Similarly patients with Aplastic anaemia (AA) treated with standard immunosuppressive therapy with ATG/ciclosporin also have increased risk of EBV reactivation but reports of PTLD in this group are very rare. The incidence of clinically significant EBV reactivation in MS patients undergoing HSCT is increasingly recognised. This retrospective study reports rates of EBV reactivation, PTLD incidence and associated clinical sequelae in patients with MS undergoing HSCT as compared with AA patients receiving ATG in our centre.
PATIENTS AND METHODS: 36 patients with MS had AHSCT between Feb 2012 and 2017. 35 were conditioned using standard protocol of cyclophosphamide and ATG (2.5mg/kg/day for 3 days), and one with BEAM-ATG. A Comparator group of 40 patients with AA consecutively treated with ATG (40mg/kg/day for 4 days) between Sept 2013 and Nov 2016 was selected. Prior exposure to EBV (EBV IgG) was assessed pre-HSCT. EBV levels monitoring was performed on whole blood samples by quantitative polymerase chain reaction (PCR) as per standard centre protocol. Data was collected retrospectively.
RESULTS: All patients had previous EBV exposure. All MS patients with relapsing remitting (n=22) and primary/secondary progressive (n=14) disease were previously treated with median 2 (range, 0-6) disease modifying therapies (DMT) and 23 had at least 1 highly active DMTs. Median age of 43yrs (range, 22-64yrs) versus 52yrs (range, 19-68yrs) in AA group, with 35 patients severe/very severe AA and 5 non-severe treated with ATG. Where follow up data was available there was no difference in EBV reactivation rates (80.6% vs 65% P-0.13). The median time to EBV detection was 28 days (IQ range, 19-41d) vs 11 days (IQ range 5-39 d)with a maximal EBV DNA titre at median of 39 days (IQ range 29.5-49.5d) vs 11.5 days(IQ Range 0-43.5) in MS and AA cohort respectively.
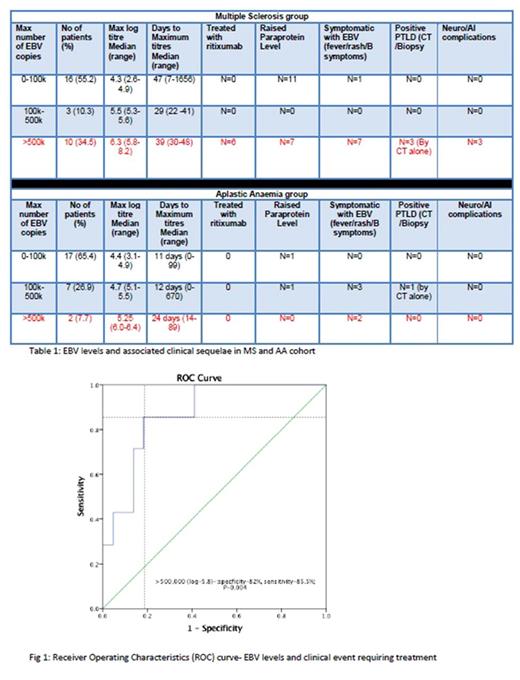
Patients were stratified into their maximum level of reactivation based on the amount of EBV copy numbers; 0-100,000 copies, 100,000 - 500,000 copies and >500,000 copies. In MS group (Table 1), 8 patients had symptomatic EBV reactivation; defined as persistent fever, lymphadenopathy and/or B symptoms. 10 (34.5%) MS patients had EBV copies>500,000. 3 of these had findings consistent with PTLD on CT scan, however none had histological diagnosis. 3 other MS patients had worsening neurological symptoms with one presenting as pseudo-relapse. All 6 of these patients were treated with anti-CD20 monoclonal therapy (Rituximab; 375mg/m2 weekly for 4 weeks) with rapid resolution of their symptoms. MS patients were more likely to develop high EBV (>500k) levels (p-0.03) and receiver operating characteristics (ROC) curve analysis (Fig 1) confirmed high EBV levels>500,000 correlate with high sensitivity 85.5%, specificity 82.5% (p-0.004) for clinical events requiring treatment. No significant rituximab related adverse events noted in treated group. As expected, no significant symptomatic EBV and no PTLD events were noted in AA cohort and none required any rituximab therapy for the EBV reactivations.
Significantly raised paraproteinaemia was observed in 21 (72.4%) of MS cohort compared to 3 (11.5%) in the AA group (p < 0.01). 3 of the MS cohort experienced paraprotein levels greater than 20 g/L and one (IgG 61.1g/L) developed hyper-viscosity requiring plasma exchange and developed neurological symptoms mimicking a MS relapse.
CONCLUSION: EBV reactivation was observed to occur commonly in patients with MS with significant symptoms and risk of neurological sequelae and PTLD events in this group, which is unlike the AA cohort treated with comparable ATG doses. Regular EBV monitoring in first 3 months should be mandated in MS patients undergoing HSCT and we recommend cut-off of 500,000 copy numbers as trigger for consideration of pre-emptive rituximab therapy.
Potter: Jazz: Honoraria; Pfizer: Other: Advisory board. Raj: Celgene: Speakers Bureau; Mallinckrodt: Speakers Bureau; MSD: Speakers Bureau; Bloodwise: Research Funding. Pagliuca: Bluebird: Honoraria; Gilead: Honoraria; Astellas: Consultancy, Speakers Bureau; Pfizer: Honoraria; Merck: Honoraria, Research Funding; Jazz: Honoraria; Basilea: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.