Abstract
Background: Immune thrombocytopenia (ITP) is an autoimmune disease mainly due to increased destruction of platelets and decreased platelet production. Prednisone 1 mg/kg/d for 2 to 4 weeks has been the standard first-line treatment for many years. Splenectomy, rituximab and cyclosporine were commonly used second-line therapies. About 20-30% ITP patients have no response to both first-line and second-line therapies or cannot maintain the response and are diagnosed with refractory ITP. There is limited evidence for the treatment of refractory ITP. The international guideline for management of ITP suggested combined therapies but about half of the patients still fail to respond. In addition, thrombopoietin-receptor agonists are not available in China. Therefore, we investigated in alternative therapeutic options for the management refractory ITP in China.
Sirolimus (rapamycin) is an immunosuppressive compound that targets the mammalian target of rapamycin (mTOR) and has been shown to induce apoptosis of both normal and abnormal lymphocytes. Recently, it has been demonstrated that treatment with sirolimus could induce complete responses (CR) in patients with corticosteroid refractory autoimmune cytopenias, including four refractory ITP patients. Based on those studies, we initiated an open-label prospective multi-institutional clinical trial (ChiCTR-ONC-17012126) using sirolimus for patients with refractory ITP in Southwest China.
Methods: Refractory ITP was defined as platelet count <50 X109/L without other complications that may cause thrombocytopenia and patient has failed prednisone therapy and at least one 2nd-line therapy (prednisone + cyclosporine / splenectomy / rituximab). Sirolimus was given at a dose of 2 mg per day orally (maximum initial dose of 4 mg/day) and target a trough level of 4-15 ng/ml in circulation. The anticipated length of treatment with sirolimus was 3 months, and then taped down 0.5mg per two weeks. Patients were followed up every week for 1 year. Platelet count was measured once a week. Complete response is defined as platelet count > 100×109/L and partial response (PR) is defined as platelet count > 100×109/L or increased more than two times. Side effects were evaluated during every visit.
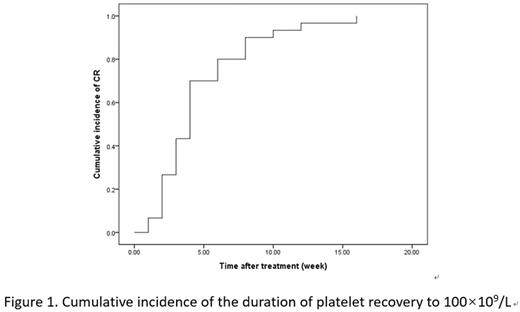
Results: Between 01/2016 and 06/2016, 73 patients were included in the trial and 66 patients completed the trial and follow-up. In 66 patients, the median age was 47 (16-68) years; 77.3% were female; 48.5% ITP patients treated with prednisone + cyclosporine, 30.3% treated with prednisone + rituximab, 13.6% treated with prednisone + splenectomy, and 7.65% treated with prednisone + two of the four second-line treatments. Fourty-six of 66 patients responded to the therapy (30 CR and 16 PR) within 3 months of sirolimus therapy. So the response rate and CR rate are 69.7% and 45.5% respectively. In patients with CR, the median duration of platelet recovery to 100×109/L was 4 weeks (Figure 1). Risk factor analysis involving age, sex, previous treatment and platelet count before sirolimus treatment were undertaken to identify whether any factor might have an effect on the outcome of sirolimus treatment. As a result, platelet count prior to treatment was the only factor that affects the efficacy of sirolimus. During sirolimus treatment and reduction, 6 patients developed oral ulcers, 8 patients with hypertriglyceridemia, 6 patients with diarrhea and 2 patients with interstitial pneumonia. All side effects were grade 1-2 according to CTCAE 4.0.
Conclusion: In summary, in our current study, more than half of the patients with refractory ITP responded to sirolimus. While the response rate is promising, there is limited evidence of side effects and long-term follow-up. Despite of this, it may worthwhile further investigations for larger scale randomized clinical trials for the evaluation of sirolimus in refractory ITP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.