Abstract
Introduction: There are no prospective clinicaltrials directly comparing results of pediatric and adult treatment approaches in adolescents with HL. We report a comparison of clinical outcomes in adolescents (14-18 years)treated within the GATLA adult clinical trial (LH05) during 2005 to 2012 due to the lack of a pediatric clinical trial in Argentina, and within the pediatric clinical trial 11-EHP-12 since 2012. In LH05 all patients (pts) were treated with ABVD and re-evaluated with PET-CT after the 3rd cycle (PET-3). Pts with Deauville Score 1-2 were considered in Complete Remission (CR) and received no further therapy. Pts with Partial Remission (PR) and PET-3 Deauville Score 3-5 continued with ABVD plus Involved Field Radiotherapy (IFRT). Pts with Stable Disease (SD) or Progressive Disease (PD) were treated with salvage chemotherapy. In 11-EHP-12 High Risk (HR) pts were treated with OEPA (vincristine, etoposide, prednisone, and doxorubicin)/COPDAC (cyclophosphamide, vincristine, prednisone, and dacarbazine) plus IFRT (20Gy:CR/25Gy:PR). Intermediate (IR) and Low Risk (LR) pts were treated with 6 or 4 cycles of ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) respectively +/- IFRT according response. Unlike the adult trial LH05, the pediatric trial 11-EHP-12 involved a more intensive therapy with dose dense chemotherapy and radiotherapy in all HR pts and IR and LR in PR.Progression-free survival (PFS) was defined as time from treatment to relapse or death from any cause. Overall survival (OS) was defined as time from treatment to death from any cause. Risk assignment: 11-EHP-12 according Stanford/Danna Farber/SJCRH Consortium classification where HR include Stages IIB, IIB and IV. LH05: Risk assigned according the International Prognostic Score (HR pts: IPS >2).
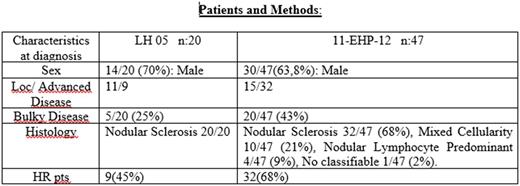
Patients and Methods : We retrospectively searched in the GATLA data base all patients with age 14-18 years treated for HL within any of the two mentioned clinical trials. Twenty pts from the LH-05 and 47 pts from the 11-EHP-12 met the inclusion criteria for this analysis.
Results: In the LH05 the EFS at 3 years was 75% vs 85% in the 11-EHP-12 with an OS at 3 years of 90% vs 93% respectively. In both groups of patients, the EFS at 3 years was lower than that of the rest the patients in the same clinical trial. In LH 05 the 3y EFS for the 377-pts analyzed was 82 % (90% for PET-3 negative and 65% for PET-3 positive) and the 3y OS was 97% (98% for PET-3 negative and 92% for PET-3 positive). In 11-EHP-12 all 120-pts analyzed had a 3y EFS: 88,5% (85% for HR, 87,7% for IR and 100% for LR) and a 3y OS of 97,4% (95,5% for HR, 100% for IR and LR). The 5 events in the Adult Trial: 4 HR pts. and 1 LR pt. 5/5 PET-3 positive.
Conclusion: Patients 14-18 years old in both clinical trials had an inferior outcome than the rest of the age groups. The EFS was superior in this group of pts treated with 11-EHP-12, although the percentage of high risk patients was higher than in the trial LH05 (68% vs 45% respectively). There was no difference in the OS. Future randomized clinical trials are needed to evaluate efficacy and safety of treating this age group with a more aggressive chemotherapy regimen and the inclusion of RT vs a conservative approach.
Pavlovsky: TAKEDA: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.