Abstract
Background:
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a progressive or recurrent neurological disorder caused by diffuse demyelination of peripheral nerves combined with muscle weakness and/or a sensory disorder. Abnormalities of both humoral and cellular immunity have been suggested to be involved in CIDP pathogenesis. But neither specific autoantibodies nor antigens have been found in patient sera. A few reports have described CIDP that develop after allogeneic stem cell transplantation (allo-SCT). However, CIDP developing after allo-SCT to treat adult T-cell leukemia-lymphoma (ATL) patients has never been described. We encountered ATL patients who developed CIDP after allo-SCT and we analyzed their disease profiles. This is the first report about CIDP in ATL patients who received allo-SCT.
Methods:
We evaluated 46 ATL patients who underwent allo-SCT. Three patients developed polyneuropathy and we performed a nerve conduction study, magnetic resonance imaging (MRI), and measurement of the levels of neopterin and CXCL10. Neopterin is a small molecule derived from guanosine triphosphate (GTP) that is produced by monocytes and macrophages in vivo, and is a sensitive marker of central nervous system (CNS) inflammation. CXCL10 is an inflammatory chemokine that binds to CXCR3 (expressed specifically on Th1 cells). CXCL10 is also a sensitive marker of CNS inflammation. Neopterin and CXCL10 are known to be elevated in CSF of patients with human T-cell leukemia virus-1 associated myelopathy (HAM). We analyzed their expression to exclude the possibility of HAM.
Results:
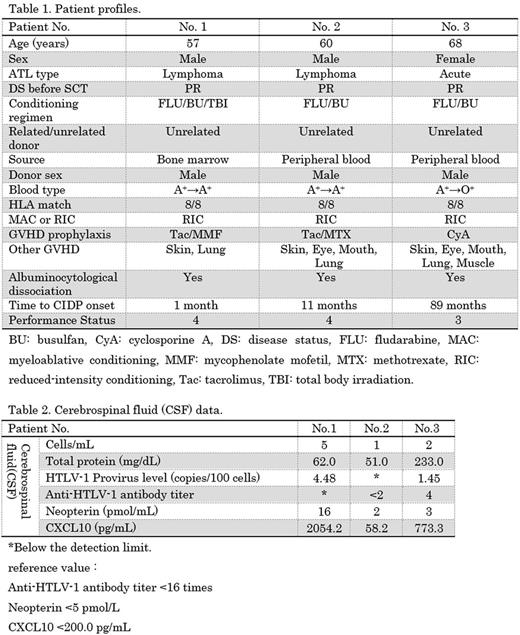
46 ATL patients, aged 28-71 years (median 59), 25 males and 21 females, we reviewed. Of these patients, three patients developed polyneuropathy after allo-SCT. Table 1 shows the patient profiles. The three patients achieved and maintained complete remission (CR) after allo-SCT but developed chronic GVHD involving multiple organ. The time of polyneuropathy onset varied from 1-89 months after allo-SCT. Table 2 shows the CSF data. A diagnosis of HAM requires that the anti-HTLV-1 antibody titer should be >16 times in both the serum and the CSF. In two patients, the CSF anti-HTLV-1 antibody titers were <2 times. In the other patient, though the anti-HTLV-1 antibody titer in the CSF was 4 times, thus weakly positive, the deep tendon reflexes were reduced and pathological reflexion was not apparent, suggesting that the patient doesn't have myelopathy. Based on these, three patients did not have HAM. Elevation of neopterin and/or CXCL10 in CSF was suggested to be related with not HAM but nonspecific immunological findings. They had progressive or recurrent neurological disorder lasting more than 2 months and diffuse demyelination of peripheral nerves proved by nerve conduction study. CSF examination showed albuminocytologic dissociation. We diagnosed three patients as CIDP according to diagnostic criteria (EFNS/PNS guidelines). Neither ATL nor HAM is likely to cause CIDP in three patients. Also, some drugs including Tac have been suggested to induce CIDP. Some patients who underwent heart or liver transplantation developed CIDP 2-10 weeks after commencing Tac. Two of our present patients were taking Tac, but the timing of CIDP onset was much later than that of other case reports. The performance status of all three patients was 3-4 and their daily life was severely disturbed. CIDP treatments include corticosteroids, intravenous immunoglobulin (IvIg), and plasmapheresis, but neither corticosteroids nor IvIg were effective in our three patients.
Conclusion
In the previous reports, chronic myelogenous leukemia, Hodgkin's lymphoma, non-Hodgkin's lymphoma, aplastic anemia, multiple myeloma, acute myelogenous leukemia, and acute lymphoblastic leukemia developed CIDP after SCT. However, CIDP has never before been reported in ATL patients who underwent allo-SCT. As far as we know, this is the first report about CIDP in ATL patients who received allo-SCT. All of our patients developed GVHD after allo-SCT. One report of CIDP after SCT found infiltrations of CD8-positive cells in nerve biopsy material. It has been suggested that CIDP may be caused by an immunological reaction developing after allo-SCT. Since ATL patients usually have immunological abnormality due to perturbed T-cell function, we need further study to elucidate whether immunological abnormality of ATL is affecting the onset of CIDP after allo-SCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.