Abstract
BACKGROUND:
Patients with renal transplants on immunosuppressive drugs are more likely to develop skin malignancies secondary to T-cell dysfunction and are at increased risk of viral reactivation. Cutaneous lymphomas are the second most common form of malignancy after skin cancer. B-cell lymphoma accounts for majority of the cases, with T-cell lymphoma accounting for 30% of the cases. T cell post-transplant lymphoproliferative disorder is usually an aggressive lymphoma. Skin lesions may be either erythematous, infiltrative plaque, nodular or ulcerative tumor. Cutaneous T-cell lymphoma expressing CD 30 generally have a good prognosis and can be treated with targeted antibody against CD30 avoiding systemic toxicity of chemotherapy. Brentuximab vedotin CD30 antibody is an antibody-drug conjugate (ADC) consisting of CD30-directed antibody, cAC10, conjugated by a protease-cleavable linker to a microtubule-disrupting agent, monomethyl auristatin E (MMAE). ADC binds to CD30-expressing cells, followed by internalization of the ADC-CD30 complex and the release of MMAE via proteolytic cleavage. MMAE then binds to tubulin to disrupt the microtubule network within the cell causing cell cycle arrest and apoptotic cell death
OBSERVATION:
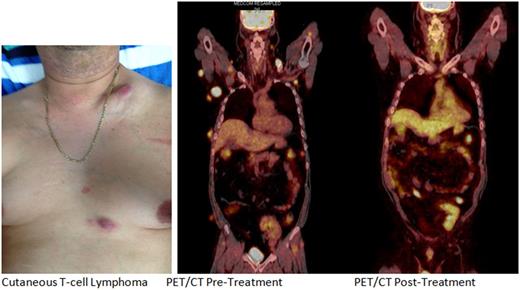
A 46 year old male received a Cadaveric Renal transplant in 1999 for presumed renal failure after Streptococcal Nephritis. Patient experienced couple of rejections and has Chronic Kidney Disease, stage III. He is maintained on immunosuppressive medication which included prednisone, Mycophenolate Mofetil and cyclosporine. Patient noticed progressive lumps in his neck, back and arms which gradually increased in size and number to occupy his front and back of neck, chest and abdomen as well as axilla. The patient did not have any constitutional symptoms. PET/CT prior to treatment revealed widespread hypermetabolic soft tissue nodules involving skin, subcutaneous fat and muscle throughout the neck, chest, abdomen and pelvis as well as anterior mediastinal lymph node consistent with malignancy. Excisional biopsy from right axillary mass showed High Grade Lymphoma in setting of post -transplant. The immunopheno type of tumor cells were positive for CD2, CD4, MUM1 and CD138 (focal, especially the larger cells). CD3 was positive mostly in apparent background lymphocytes. CD30 was strongly and diffusely positive. The malignant cells were negative for EBV, CD79A, ALK-1 and CD20. The complex immunophenotype was consistent with the diagnosis of monomorphic post-transplant lymphoproliferative disorder of T lymphocytes. The dose of immunosuppressive drug was reduced and the patient was treated with Brentuximab vedotin CD30 antibody - drug conjugate 1.2 mg/kg every 3 week (Dose adjusted for renal impairment). The patient tolerated the treatment very well with mild grade 1 peripheral sensory neuropathy localized to bilateral finger tips treated with Pregabalin. Repeat PET/CT after six cycles of treatment with Brentuximab vedotin showed interval resolution of previously described hypermetabolic soft tissue nodules and anterior mediastinal lymph node. No evidence of residual/recurrent hyper-metabolic foci. The patient continues to be on Brentuximab vedotin every three week now after 6 months of treatment.
CONCLUSION:
CD30+ Monomorphic cutaneous T-cell lymphoma occurring in post renal transplant patients with renal impairment and on immunosuppressive medication can be successively treated with targeted drug Brentuximab vedotin with dose adjustment with excellent response as in this case. Brentuximab Vedotin should be avoided in severe renal failure (Creatinine Clearance< 30 mlL/min) due to higher risk of Grade 3 adverse reactions. Brentuximab vedotincan be safely use with dose modification and close observation for toxicity in patients with moderate renal impairment and post renal transplant setting.
D'Cunha: Seattle Genetics Inc.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.