Abstract
Introduction: Chronic Myeloid Leukemia (CML) represents 20% of adult leukemia. Tyrosine kinase inhibitors (TKI) of BCR-ABL, are the standard first-line therapy. Imatinib was the first TKI approved, however, a percentage of patients will present a suboptimal response or will develop resistance. Dasatinib has 325-fold increased potency over imatinib against BCR-ABL kinase in vitro and has activity against most of the mutated forms that have been associated with clinical resistance to imatinib. The approved dose in chronic phase is 100mg once a day and 140mg in accelerated phase. Lower doses of dasatinib have been successfully used and reported in intolerant patients so doubts arise if lower doses can reduce adverse effects and maintain anti-leukemic effect. In our country medical insurance cover is not universal, thus many patients cannot access to ITK treatment easily. We aim to demonstrate that 50mg of dasatinib is as effective as the full dose to induce molecular response as first line therapy in CML.
Methods : We performed a single-arm open label phase II study. All patients with newly diagnosed non-previously treated chronic-phase CML were included. They receive dasatinib 50mg once daily for 6 months. They were evaluated weekly the first two weeks and then monthly. Initial, 3 and 6 months BCR/ABL p210 polymerase chain reaction (PCR) were measured. We documented timing to obtain hematological response defined by a white blood cell count (WBC) <10,000/mL with no immature granulocytes and <5 percent basophils on differential; platelet count <450,000/microL; and spleen not palpable. Major molecular response (MMR) was defined as a ≥3-log reduction in BCR-ABL1/BCR ratio and was measured at 3 and 6 months. Adverse effects were documented.
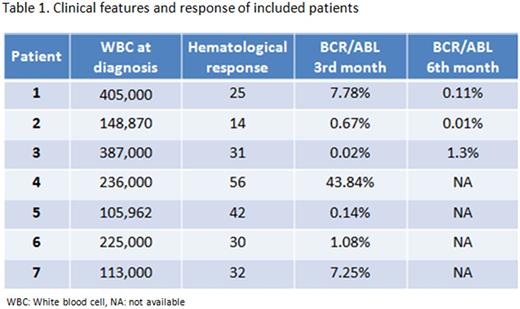
Results: Seven patients have so far been included (5 males and 2 females). Mean age was 43 years (range, 16-73). The median WBC at diagnosis was 231,547 x 10^3/µL (range, 105,962-405,000). The hematological response was achieved in a median of 32 days (range, 14-56). Six patients (85%) had optimal response, including two with minor molecular response and one with mayor molecular response. Until now, only 3 patients have completed the 6th month evaluation, and two (66%) had achieved MMR. None presented any grade 3 or 4 adverse effects, and only one case of rash grade 1.
Conclusions: Until now the use of low-dose dasatinib had satisfactory results including two major molecular response and no major adverse effects. It is an interesting option for patients with low tolerance or in centers with limited resources.
Gomez-Almaguer: Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.