Abstract
Background
Gene therapy with LentiGlobin Drug Product (DP), which contains autologous CD34+ hematopoietic stem cells transduced with the betibeglogene darolentivec (BB305) lentiviral vector, has been shown to eliminate red blood cell (RBC) transfusions in most patients with TDT with non-β0/β0 genotypes in the prior HGB-204 (Northstar) phase 1/2 study, with a safety profile consistent with myeloablative conditioning. A key finding in HGB-204 was that the average number of therapeutic gene copies per CD34+ cell (i.e. vector copy number [VCN] per diploid genome; median 0.7, range 0.3-1.5) in the DP correlated with peripheral HbAT87Q (transgenic hemoglobin [Hb]) expression at 6 months. To optimize the proportion of patients able to achieve "transfusion independence" and increase unsupported Hb levels after treatment, a refined manufacturing process for LentiGlobin DP was used, to increase the DP VCN and the proportion of transduced cells. Herein, we report initial data from the phase 3 HGB-207 (Northstar-2) trial, recently initiated to evaluate safety and efficacy of autologous transplantation with LentiGlobin DP, using this refined manufacturing process, in patients with TDT and a non-β0/β0 genotype (NCT02906202).
Methods
The HGB-207 study is enrolling patients (12-50 years old) with TDT (≥100 mL/kg/year of packed RBCs or ≥8 pRBC transfusions/year) and a non-β0/β0 genotype. Following mobilization with G-CSF + plerixafor, CD34+ cells are harvested via apheresis. After individualized DP manufacture, patients receive myeloablative conditioning with single-agent busulfan, followed by infusion of LentiGlobin DP. Patients are followed for 2 years after infusion and can then be enrolled in a 13-year follow-up study. The primary endpoint is proportion of patients who achieve transfusion independence after DP infusion, defined as a weighted average Hb ≥9g/dL without pRBC transfusions for ≥12 months continuously. Additional endpoints include time to engraftment, adverse events (AEs), and biological parameters, including peripheral blood VCN and HbAT87Q levels over time.
Results
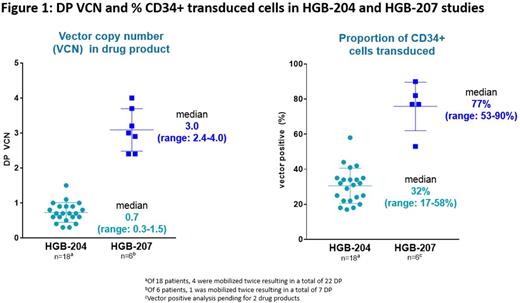
As of June 2017, 17 patients have been consented; mobilization and DP manufacturing has been completed for 6 patients. DP VCN and percent CD34+ transduced cells in these 6 patients were consistently higher than those observed in the 18 patients treated in the initial HGB-204 study (Figure 1). Three female patients have been treated with LentiGlobin DP, including two 20-year-olds with β0/βE and one 22-year-old homozygous for IVS-I-5 (G>C) β+ mutation. These 3 patients had pre-study pRBC transfusion volumes of 159-193 mL/kg/year and 2 had substantial liver iron overload at baseline. At a median follow-up of 3 (range 2-6) months, all 3 treated patients had successful engraftment; no significant veno-occlusive disease of the liver or infections and no DP-related AEs were observed. Grade ≥3 non-hematologic AEs were observed in 2 patients: hypotension (considered serious) and epistaxis in 1 and mucositis in the other. Total Hb; HbAT87Q; and peripheral blood VCN in the 3 patients with 6, 3 and 2 months follow-up was 13.3, 8.4 and 11.2 g/dL; 9.5, 1.6 and 4.6 g/dL; and 3.4, 0.3 and 2.2, respectively. The first patient treated has been transfusion-free for 5 months. Since the time of this data cut, 2 more patients have been treated.
Summary
The refined manufacturing process used in the Northstar-2 trial yields, on average, DPs with higher VCNs and proportion of transduced cells. These improved DP parameters may translate into higher in vivo VCN and HbAT87Q production than has been previously seen while maintaining a safety profile consistent with myeloablative conditioning. Longer follow-up on the 3 treated patients and data from approximately 5 additional treated patients will be presented.
Walters: bluebird bio: Research Funding; Sangamo Therapeutics: Consultancy; AllCells, Inc: Other: Medical Director; ViaCord Processing Lab: Other: Medical Director. Kwiatkowski: Agios: Consultancy, Honoraria; Novartis: Research Funding; Ionis: Consultancy, Honoraria; Apopharma: Research Funding; Bluebird Bio: Research Funding. Porter: Novartis: Consultancy, Honoraria, Research Funding; Bluebird Bio: Consultancy; Celegene: Consultancy; Agios Pharmaceuticals: Consultancy, Honoraria; Shire: Consultancy, Honoraria. Sauer: Neovii: Research Funding. Thrasher: Torus Therapeutics, Inc: Other: Advisory Board; Orchard Therapeutics: Consultancy; 4bio Ventures Management Ltd: Other: Advisory Board; Rocket Pharmaceuticals Ltd: Consultancy. Elliot: bluebird bio: Employment, Equity Ownership. Gayron: bluebird bio: Employment, Equity Ownership. Asmal: bluebird bio: Employment, Equity Ownership. Thompson: bluebird bio: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Baxalta: Research Funding; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract