Abstract
The antiphospholipid syndrome (APS) is characterized by thrombosis and/or specific pregnancy morbidity with the persistent presence of antiphospholipid antibodies (aPL). Laboratory criteria include aPL detection by a functional assay referred to as lupus anticoagulant (LAC) or by quantitative solid phase assays measuring anti-β2glycoprotein I (aβ2GPI) and anti-cardiolipin (aCL) IgG/IgM antibodies. Triple positivity (i.e. positivity for LAC, aCL and aβ2GPI antibodies irrespective of the isotype) is associated with a high risk for both a first thrombotic event and recurrence. For these high-risk triple positive patients, a repetitive aPL measurement proved not to be required. Reports from external quality control programs illustrate that commercially available aPL assays produce variable results, probably originating from differences in assay design, epitope exposure and a lack of standardization. On the contrary, the detection of triple positivity was recently suggested to be method and platform independent. In a large multicentre study, we aimed to investigate the variability in triple positivity detection and its clinical association between different aPL platforms.
In this study we included 853 patients from seven European medical centers. Patients were classified by the local centers resulting in 259 thrombotic APS patients, 204 diseased controls for thrombosis, 196 patients with autoimmune diseases other than APS and 194 patients without any clinical and laboratory criteria for APS. Four platforms, ACL AcuStar (Instrumentation Laboratory, Bedford, MA, USA), Bioplex 2200 (Bio-Rad Laboratories, Hercules, CA, USA), ImmunoCap EliA (ThermoFisher (Phadia), Waltham, MA, USA) and QUANTA Lite ELISA (Inova Diagnostics, San Diego, CA, USA), were selected based on frequency of use and the willingness of manufacturers to provide their assays. All samples with all assays were tested at one location by a single technician. Lupus anticoagulant positivity was determined by the local center, according to the ISTH guidelines.
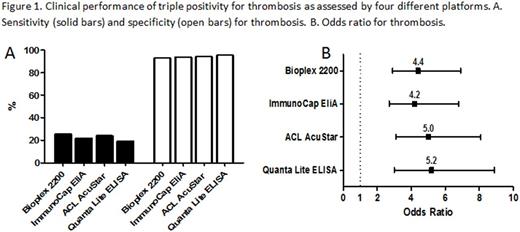
From the 259 patients diagnosed with thrombotic APS, 202 were positive for LAC. Detection of triple positivity proved to be variable between platforms: 118, 101, 111 and 89 detected with Bioplex, ImmunoCap EliA, AcuStar and QUANTA Lite respectively. To investigate the diagnostic efficacy of triple positivity of the different platforms, we calculated the sensitivity, specificity and odds ratio (OR) for thrombosis. The sensitivity of triple positivity for thrombosis was low and varied from 19% up to 26% between the tested platforms (Figure 1A). However, a high specificity for thrombosis with minor variation from the platforms was obtained ranging from 93% up to 96% (Figure 1A). Looking at the OR for thrombosis, triple positivity was significantly correlated with thrombosis independent of the platform used (Figure 1B). However, the OR for thrombosis ranged from 4.2 up to 5.2 for the different platforms used to detect aβ2GPI and aCL aPL.
Taken together, our study confirmed the high-risk profile for thrombosis associated with triple positivity, independent of the platform used to detect aβ2GPI and aCL aPL. Contrary, the number of triple positives is dependent on the platform used, resulting in varying sensitivities of triple positivity for thrombosis over the different platforms. The high risk on recurrent clinical complications in triple positive APS patients often requires them to take indefinite oral anticoagulant treatment. Therefore, to avoid misclassification of high-risk APS patients, standardization/uniformity of solid phase assays for the diagnosis of APS is urgently warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.