Abstract
Multiple myeloma (MM) is largely incurable because relapse eventually occurs, despite the recent development of novel therapies. This is mainly caused by a remaining population of drug-resistant myeloma stem cells. There is a side population (SP) enriched with myeloma stem cells. Therefore, targeting SP cells may be a promising strategy to prevent and treat MM relapse.
Polycomb repressive complexes 1 (PRC1) and 2 (PRC2) are important epigenetic regulators that maintain the "stemness" of embryonic and hematopoietic stem cells. Enhancer of zeste homolog 1 and 2 (EZH1/2) are catalytic components of PRC2, which trimethylate histone H3 at lysine 27 (H3K27me3) to repress transcription of target genes. Mutation and overexpression of EZH2 are associated with many cancers, including MM. Here, we found that expression of EZH1/2 was significantly higher in SP cells than in non-SP cells. These results suggest that overexpression of EZH1/2 is important for maintaining the stemness of MM cells and that both EZH1 and EZH2 are potential therapeutic targets.
We developed a novel EZH1/2 dual inhibitor, OR-S1, which potently inhibited both EZH1 and EZH2, and used it to investigate the effect of pharmacologic inhibition of EZH1/2 on MM. OR-S1 suppressed the proliferation of all MM cell lines tested, and its GI50 value was significantly lower than that of the EZH2-specific inhibitor GSK126. Furthermore, flow cytometric analysis revealed that treatment with OR-S1 significantly depleted the SP cells. These results suggest that dual inhibition of EZH1/2 using OR-S1 eradicates myeloma stem cells.
RNA sequencing analysis revealed that the transcriptional profiles of OR-S1-treated MM cell lines were characterized by up-regulation of genes related to the Wnt pathway. RT-PCR confirmed that OR-S1 treatment markedly increased expression of Wnt and Frizzled family members. Furthermore, western blot analysis revealed that non-phosphorylated (active) β-catenin was strongly expressed in OR-S1-treated cells. To determine whether there is direct crosstalk between PRC2 and Wnt signaling, we performed chromatin immunoprecipitation (ChIP)-sequencing using MM cell lines. This revealed that most Wnt and Frizzled loci were marked by H3K27me3. Furthermore, ChIP-qPCR analysis demonstrated that dual inhibition of EZH1/2 using OR-S1 markedly decreased the level of H3K27me3 at most of these loci. These results demonstrate that PRC2 directly targets Wnt signaling and that dual inhibition of EZH1/2 using OR-S1 activates canonical Wnt signaling.
Increased activation of the canonical Wnt signaling pathway reduces self-renewal and differentiation of hematopoietic stem cells. Therefore, we generated β-catenin-overexpressing MM cells to examine the effects of increased Wnt signaling. Surprisingly, proliferation of these cells was significantly lower than that of control cells. Furthermore, flow cytometric analysis revealed that overexpression of β-catenin significantly depleted the SP cells. These results suggest that over-activation of Wnt signaling induced by dual inhibition of EZH1/2 reduces the proliferation of MM cells by inhibiting self-renewal, which is important to maintain the stemness of MM cells.
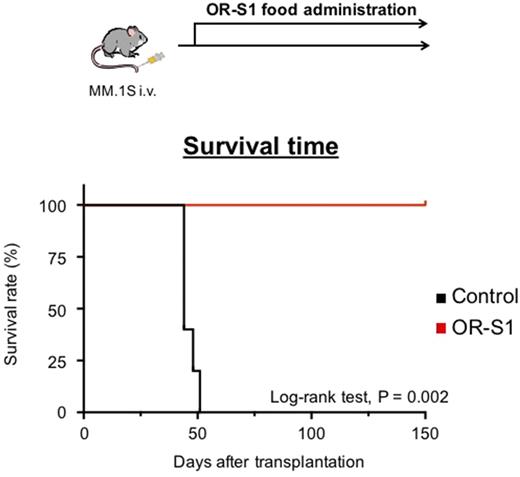
Oral administration of OR-S1 to mice bearing MM xenografts significantly impaired subcutaneous tumors. Long-term administration of OR-S1 at lower doses to mice bearing orthotopic xenografts eradicated minimal residual disease from bone marrow and completely cured MM, without eliciting any serious side effects (Figure). Furthermore, we developed an orthotopic patient-derived xenograft model using specimens from a relapsed and heavily pretreated MM patient. In this model, OR-S1 treatment reduced the serum levels of human immunoglobulins. These results indicate that EZH1/2-targeting therapy with OR-S1 is also effective against a human MM xenograft.
Taken together, our results strongly suggest that dual inhibition of EZH1/2 is a promising therapeutic approach to eradicate myeloma stem cells and could lead to important advances in the treatment of MM.
Nakagawa: Daiichi Sankyo Co.: Research Funding. Fujita: Daiichi Sankyo Co.: Research Funding. Honma: Daiichi Sankyo Co., Ltd.: Employment. Araki: Daiichi Sankyo: Employment. Kitabayashi: Daiichi Sankyo Co.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.