Abstract
Introduction: Maintenance and/or improvements in health-related quality of life (HRQoL) are important in patients with chronic lymphocytic leukemia (CLL). GIBB (NCT02320487) is an ongoing, open-label, single-arm Phase II study of the combination of obinutuzumab (GA101; G) and bendamustine (B) (BG) in patients with previously untreated CLL. A previous report from the GIBB study demonstrated an investigator-assessed objective response rate of 89.2%, a complete response rate of 49.0%, and no unexpected safety signals with BG (Sharman et al. ASCO 2017). Here, we present the HRQoL data from GIBB.
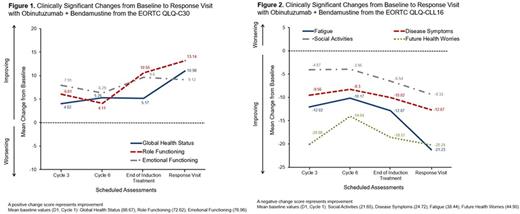
Methods: In the GIBB trial, patients received BG by intravenous infusion over six 28-day cycles: obinutuzumab 100mg on Day (D)1, 900mg on D2, and 1000mg on D8 and D15 of Cycle 1, then 1000mg on D1 of Cycles 2-6; B 90mg/m2 on D2-3 of Cycle 1, and on D1-2 of Cycles 2-6. The European Organisation for Research and Treatment of Cancer Quality of Life - Core (EORTC QLQ-C30) questionnaire includes a global health status measure, 5 functional scales (physical, emotional, cognitive, social, and role functioning), 8 symptom scales/items (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, and diarrhea), and an item on financial difficulties (Aaronson et al. J Natl Cancer Inst 1993). The EORTC Quality of Life Questionnaire-Chronic Lymphocytic Leukemia 16 (QLQ-CLL16) is a 16-item module, specific to CLL, containing 4 multi-item scales (fatigue, treatment side effects, disease symptoms, and infection) and 2 single items (social activities and future health worries; EORTC website, accessed July 25, 2017). Both questionnaires were completed by patients on D1 of Cycles 1 (baseline), 3, and 6, at the end of induction treatment (defined as +28 days from D1 of Cycle 6 or early treatment termination visit), at the response visit (defined as 2-3 months after the end of induction treatment, for all patients who received study treatment and had not experienced disease progression), and every 3 months thereafter at follow-up visits. HRQoL scores were linear transformed to a 0-100 point scale. Mean baseline scores and mean score changes at each visit were evaluated. A threshold of ≥10-point change in score represents a clinically meaningful difference.
Results: Of 102 patients enrolled in the trial, 98 completed a questionnaire at baseline and at least one other questionnaire during a follow-up visit. Questionnaire completion rates were 86.7%, 77.6%, 80.6%, and 86.7% at Cycles 3, 6, at the end of induction treatment, and at the response visit, respectively. Median age was 61 years and 68.4% of patients were male. According to the EORTC QLQ-C30 (Figure 1), clinically meaningful improvements were observed for global health status at the response visit, and for role functioning at the end of induction treatment and at the response visit. A trend was observed for improvement in emotional functioning. The greatest improvement in HRQoL score was observed for fatigue (mean baseline score: 37.64), with mean changes from baseline of −4.01, −5.48, −11.67, and −16.34 at Cycles 3, 6, at the end of induction treatment, and at the response visit, respectively. Improvements were also observed for insomnia (mean baseline score: 33.33), with mean changes from baseline of −6.59, −9.09, −9.7, and −10.98, respectively. There was no worsening in other patient-reported symptoms or functional status over time. Similarly, with the EORTC QLQ-CLL16 (Figure 2), clinically meaningful improvements in symptoms were observed for fatigue, disease symptoms, and future health worries during treatment, at the end of induction treatment and/or at the response visit. The greatest change at the response visit was observed for fatigue (−21.23) and future health worries (−20.24). A positive trend was also observed for improvements in the social activities scale.
Conclusions: We previously reported that BG is an effective regimen for first-line treatment of CLL with no unexpected safety signals. In addition, the HRQoL data from the GIBB trial suggest that BG treatment improves patient HRQoL. Several clinically meaningful improvements were observed in HRQoL, including global health status, functioning, symptoms, and future health worries at the time of the response visit.
Sharman: Acerta: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Genentech: Consultancy, Honoraria, Other: GIBB is sponsored by Genentech Inc. Third-party editorial support, under the direction of Anthony Masaquel, was provided by Lynda McEvoy of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd, Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Yimer: Juno pharma: Equity Ownership; Bellucum Pharma: Equity Ownership. Babu: Alexion: Speakers Bureau; Abbvie: Consultancy. Li: Genentech: Employment, Equity Ownership. Mun: Genentech: Employment, Equity Ownership. Trask: Genentech: Employment, Other: stock. Masaquel: Genentech Inc.: Employment, Other: I Receive Roche stock options. Reyes: Genentech Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.