Abstract
Background: Recurrent joint bleeding in severe congenital hemophilia results in arthropathy and functional impairment. Clinical and epidemiologic evidence suggest that patients with moderate and mild hemophilia also experience joint bleeding, particularly with factor activity (FA) levels below 15-20%. While arthropathy and joint interventions have been reported in mild-moderate hemophilia, the longitudinal assessment of arthropathy development and relationship to FA has not been reported.
Methods: During the Centers for Disease Control and Prevention (CDC) Universal Data Collection (UDC) surveillance initiative (1998-2011), joint range of motion (ROM) measurements were taken on each of 10 joints (shoulders, elbows, hips, knees and ankles) by trained care providers using standardized methods at each comprehensive visit. Data were extracted from male patients with hemophilia (PWH) age ≥2 years with baseline FA levels ≤ 40%, excluding those who had been prescribed prophylaxis or had evidence of an inhibitor at any time. ROM measures from all 10 joints combined for each subject and data collected similarly on a population without bleeding or joint disorders (Soucie JM, Haemophilia 2012) age 12-20 males) were used to calculate a proportion of normal ROM (PN-ROM) measure for each study subject and each normal male using the 12-20 year old normals as the reference. Because very young subjects have greater ROM than 12 - 20 year olds, the PN-ROM value for these subjects could exceed 100%. Least square means of the PN-ROM values for subjects in categories of these characteristics were compared using general linear regression. Data collected from 2 to 14 UDC visits for each subject were analyzed using mixed model repeated measures linear regression to evaluate the effects of patient characteristics on the rate of ROM loss over time.
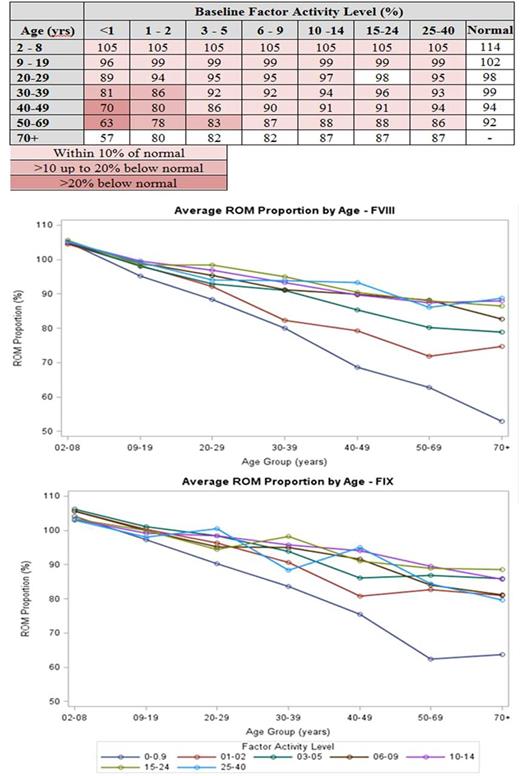
Results: There were 6,703 (4,807 hemophilia A) eligible PWH with 30,102 UDC visits (mean 4.5 per patient). Of these, 26% had severe and 31% moderate hemophilia, 52% were youth or teens, 10% were either black or Hispanic, and 45% were overweight or obese. PN-ROM declined with age (106% for youngest to 85% for oldest subjects), and was associated with hemophilia severity, race/ethnicity, obesity, and viral illnesses. The relationship between PN-ROM and the combination of age and baseline FA level (Table) showed values for most PWH were within 10 percent of similarly aged normals. Only PWH ≥30 years old with FA ≤2% and those ≥50 years old with FA ≤5% had mean PN-ROM values >10% less than controls; those ≥40 years old with FA <1% had PN-ROM values >20% less than controls.
The figures demonstrate that the loss in PN-ROM is linear with the steepest decline among subjects with severe disease, and the overall magnitude of the decline appears to be greater for subjects with hemophilia A than B.
In the multivariate analysis subjects with <1% FA had a 0.428 percent greater decrease in PN-ROM each year relative to those with 16% - 40% FA and this excess decrease was highly statistically significant (p < 0.001). A similar significant effect was seen among subjects with either 1% - 5% or 6% - 9% FA, however, the magnitude of the decrease in the PN-ROM (0.126 for both) was about one-fourth that seen among those with severe hemophilia. FA levels from 10% to 15% did not significantly influence the rate of PN-ROM change over time relative to those with FA >15%. Those with hemophilia B lost PN-ROM at a 0.05 percent slower rate than those with hemophilia A (p < 0.001).
Conclusion: The effect of FA level on ROM loss is far greater than that of any of the other characteristics, but only for patients with FA levels less than 10%. This emphasizes the need to maintain a high index of suspicion in individuals with moderate and low-mild hemophilia and of older age. The effect of hemophilia type (A vs B) on rate of ROM loss is about one-tenth that of having severe disease, and may be one reason for the difficulty in proving that hemophilia B has a less severe phenotype.
Wang: Acerta Pharma: Consultancy, Research Funding; Asana Biosciences: Research Funding; BeiGene: Research Funding; Celgene: Honoraria, Research Funding; Dava Oncology: Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; June Therapeutics: Research Funding; Kite Pharma: Research Funding; Onyx: Research Funding; Pharmacyclics: Research Funding; Proteolix: Honoraria, Research Funding. Recht: Biogen: Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Kedrion: Membership on an entity's Board of Directors or advisory committees; NovoNordisk: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees, Research Funding. Iyer: Novo Nordisk Inc.: Employment. Cooper: Novo Nordisk Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.