Abstract
Allogeneic hematopoetic stem cell transplantation (HCT) offers an option for patients with hematologic malignancies, in whom conventional standard therapies failed or are not effective enough to cure the disease. Successful HCT can restore functional hematopoiesis and immune function, and the new donor-derived immune system can exert a graft-versus-leukemia (GVL) effect. However, allogenic HCT can also be associated with serious risks for transplantation-related morbidities or mortalities such as graft-versus-host disease (GVHD) or life-threatening infectious complications. GVHD is caused by alloreactive T lymphocytes, which express the αβ T-cell receptor, whereas lymphocytes expressing the γδ T-cell receptor are not alloreactive and do not induce GVHD but can exhibit potent antileukemia and anti-infectious activities. Therefore, γδ T cells are becoming increasingly interesting in allogeneic HCT, and clinical strategies to exploit the full function of these lymphocytes have been and are being developed. Such strategies comprise the in vivo activation of γδ T cells or subsets after HCT by certain drugs or antibodies or the ex vivo expansion and manipulation of either patient-derived or donor-derived γδ T cells and their subsets and the adoptive transfer of the ex vivo–activated lymphocytes. On the basis of the absence of dysregulated alloreactivity, such approaches could induce potent GVL effects in the absence of GVHD. The introduction of large-scale clinical methods to enrich, isolate, expand, and manipulate γδ T cells will facilitate future clinical studies that aim to exploit the full function of these beneficial nonalloreactive lymphocytes.
Introduction
γδ T cells are a unique and conserved population of lymphocytes that, as a first line of defense, mediate innate immunity against a wide variety of infections and play unique roles in immune surveillance and tissue homeostasis. Because of their ability to kill tumor cells of various origins, including leukemic blasts, and the fact that allogeneic T-cell receptor (TCR) γδ T cells, in contrast to allogeneic TCR αβ T cells, are not alloreactive and do not cause graft-versus-host disease (GVHD), γδ T cells are increasingly the focus of research for adoptive immunotherapy in the context of allogeneic hematopoetic stem cell transplantation (HCT). γδ T cells are part of the innate lymphocyte family and differ from conventional αβ T cells by the absence of CD4 and CD8 coreceptors. Their TCR heterodimer consists of a γ chain and a δ chain, each encoded with a restricted repertoire of V, D, and J gene segments, rearranged with the help of recombination activating genes. According to their TCRδ chain usage, human γδ T cells are generally divided into 2 major subsets, namely Vδ1 and Vδ2.1 Although Vδ1+ T cells are predominantly associated with a γ chain of the Vg I gene family comprising Vγ2/3/4/5/8, the majority of Vδ2+ T cells express an invariant TCR harboring Vγ9.2 A small subset of Vδ3+ T cells has been described, which might play a role in immunity against cytomegalovirus (CMV).3 In humans, γδ T cells account for 1% to 20% of all CD3+ circulating T lymphocytes but constitute the major subset of resident T cells in skin and mucosa.4,5 Whereas the Vγ9δ2 TCR is expressed by 50% to 95% of the peripheral γδ T cells, TCR including other Vδ elements are predominantly found in the epithelia and the skin. On activation, γδ T cells can respond to signals from microbes or tumors with the production of Th1, Th2, Th17, and other (inflammatory) cyto- and chemokines and interact with dendritic cells (DCs). Vγ9Vδ2 T cells exhibit a potent antigen-presenting capacity and can prime CD4+ and CD8+ T cells more efficiently than do DCs.6-8 In this review, we describe physiological and manipulated antileukemic cytotoxic mechanisms of γδ T cells and how their antileukemic properties can be exploited posttransplant in patients with leukemia.
Antitumor cytotoxicity of γδ T cells
Physiological antigen recognition of γδ T cells
γδ T cells can exert effective antitumor activity against various solid tumors9 and hematologic malignancies, such as lymphoma10 and multiple myeloma.11 In contrast to αβ T cells, γδ T cells are not HLA-restricted for antigen recognition, and their activation does not require antigen processing and presentation by antigen-presenting cells (APCs). Therefore, γδ T cells can be rapidly activated during the early phase of an immune response. Very similar to natural killer (NK) cells, they can respond to stress-induced self-ligands such as major histocompatibility complex class I–related chain A and B (MICA/B) and UL16-binding proteins (ULBPs) via activation of the NKG2D receptor expressed on γδ T cells and via the Vδ1 receptor.12-15 Some of the NKGD2 ligands can directly engage the γδ TCR in Vδ2 T cells.16 In addition, γδ T cells also express pattern-recognition receptors, such as toll-like receptors, which augment their antitumor activity.17 It has also been shown that γδ T cells express natural cytotoxicity receptors NKp30 and NKp44 and that γδ T cells can kill lymphoid leukemia cell lines and leukemic blasts from patients with chronic myeloid leukemia through NKp30.18 Finally, with the activating receptor DNAM-1, γδ T cells efficiently target nectin-2 (CD112) and poliovirus receptor (CD155) positive acute myeloid leukemia and multiple myeloma cells.19,20 Upregulation of Fas ligand and TRAIL through TCR activation also leads to enhanced tumor killing.21 γδ T cells recognize a ligand repertoir heterogeneous in size, composition, and molecular structure, mainly including non–major histocompatibility complex (MHC) cell surface molecules, soluble proteins, and smaller peptides, phospholipids, prenyl pyrophosphates, and sulfatides that are bound to or expressed on the cell surface.22 Through their TCR, peripheral Vδ2 T cells recognize nonpeptidic phosphorylated antigens, so-called phosphoantigens (PAgs). Some natural phosphoantigens such as (E)-4-hydroxy-3methyl-but-2-enylpyrophosphate—intermediates of the non-mevalonate isoprenoid pathway in bacteria—are strong activators of Vγ9Vδ2 T cells23,24 ; others, such as isopentenyl pyrophophate (IPP), are produced during metabolic processes in eukaryotic cells via the mevalonate isoprenoid synthesis pathway. Although physiologic levels of IPP do not activate γδ T cells, the increased metabolism of tumor cells does, by producing increased levels of PAgs, which can be recognized by γδ T cells as tumor-associated antigens.25
Pharmacological ways of stimulating, activating, and modifying γδ T cell responses
In vivo stimulation of Vδ2 γδ T cells can be achieved by using aminobisphosphonates (eg, zoledronate, pamidronate, risedronate), which as inhibitors of the farnesyl pyrophosphate synthetase (FPPS) in the mevalonate pathway provoke the accumulation of IPPs and thus activation of γδ T cells in vivo. A direct correlation between bisphosphonate-induced FPPS inhibition, and thus accumulation of PAgs and tumor recognition by Vγ9Vδ2 T cells, has been shown for more than 50 tumor cell lines for zoledronate26 as well as for risedronate.27 A comprehensive analysis of 19 primary acute myeloid leukemia (AML) samples by Gundermann et al correlated genetic, metabolic, and immunophenotypic features with the recognition of the blasts by allogeneic Vγ9Vδ2 T cells and found that an intrinsic susceptibility for γδ T cell recognition was associated with ULBP1 expression, whereas pretreatment of nonsusceptible blasts with bisphosphonates increased their susceptibility for lysis by γδ T cells.28 Noteworthy, in the absence of accessory cells, nucleotide phosphoantigens as precursors of pyrophosphate antigens can deliver strong agonists intracellularly when transformed into strong pyrophosphate agonists by nucleotide pyrophosphatase activity present in serum, resulting in prolonged and strengthened activity.29 Gerner-Dardenne et al showed high cytolytic potential toward AML blasts via TCR- and DNAM-1 activation for in vitro stimulated Vγ9Vδ2 T cells.19 In vitro cytotoxicity of γδ T cells against acute lymphoblastic leukemia (ALL) blasts was demonstrated by Lamb et al.30 It was shown in vitro in an allogeneic transplant setting that donor-derived γδ T cells were activated and proliferated in response to recipient ALL blasts, whereas the γδ T cells did not proliferate in a mixed lymphocyte culture with HLA-mismatched stimulator cells. In addition, the proliferating γδ T cells were highly cytotoxic against myeloid and lymphoid target cells.
More recently, an emerging role of butyrophilins (BTNs) in γδ T cell activation has been described31 . BTNs are a large family of proteins and members of the extended B7 family of costimulatory molecules.32 CD277/butyrophilin 3 (BTN3A1) was identified as a crucial molecule indispensable for activation of Vγ9 T cells by PAgs.33 In a mechanism termed “inside-out signaling,” Vγ9Vδ2 TCR activation is modulated by the guanosine triphospatase activity of RhoB and its redistribution to BTN3A1. This stabilizes BTN3A1 in the membrane, and IPP accumulation induces a conformational change of BTN3A1, which leads to recognition of the extracellular domains by Vγ9Vδ2 TCRs34 . This mechanism is reported to be active on leukemic stem cells but not healthy CD34+ stem cells.34
Accordingly, anti-BTN3A1 antibodies can mimic phosphoantigen stimulation and selectively activate Vγ9 T cells, whereas inhibitory anti-BNT3A1 antibodies can neutralize PAg stimulation. Incubation of AML blasts with anti-BNT3A1 triggered BTN3A1 on the blasts and resulted in an enhanced Vγ9Vδ2 T cell–mediated killing and could also specifically sensitize resistant blasts to Vγ9Vδ2 T cell lysis. Furthermore, in a preclinical xenotransplantation model of AML, application of the agonistic anti-CD277/BTN3A1 antibody mAb 20.1 enhanced the therapeutic efficacy of adoptively transferred Vγ9Vδ2 T cells.35
Another key mode of antitumor activity is the activation of γδ T cells via the Fc receptor FcRγIII (CD16). This Fc receptor, by binding to the Fc region of immunoglobulins (IgG), can induce tumor cell lysis via antibody-dependent cellular cytotoxity (ADCC) in the presence of antibodies that target and bind antigens expressed by the tumor.36 Stimulation of γδ T cells with phosphoantigens leads to upregulation of CD16 and increased ADCC,37 and antibodies against CD20 or HER2/neu have been shown to enhance the cytotoxicity of γδ T cells against tumor cells expressing these target antigens.38 More recently, Seidel et al presented data on ADCC by freshly isolated as well as in vitro expanded γδ T cells against ALL blasts in the presence of an Fc-optimized anti-CD19 antibody.39 Similar results were reported by Schiller et al, who generated triple bodies ds(19-16-19).40 These triple bodies have 2 binding sites for CD19 and 1 for CD16 and are able to mediate a very rapid lysis of CD19-positive leukemic blasts mediated by primary and in vitro expanded γδ T cells.
The role of γδ T cells in allogeneic HCT
On the basis of the observations that γδ T cells are not HLA-restricted for antigen recognition, these cells might offer the opportunity for graft-versus-leukemia (GVL) effects in the absence of GVHD. Therefore, the detailed analysis of the reconstitution of γδ T cells and their subsets, their in vivo activation, and the adoptive transfer of γδ T cells after allogeneic transplantation are areas of increasing interest.41 Whereas earlier reconstitution studies showed increased numbers of γδ T cells in patients who developed acute GVHD up to 3 months after allogeneic HCT,42 later studies could not corroborate these findings, and no significant correlation between γδ T cell recovery and the incidence of GVHD in the first 12 months posttransplant was found by Cela et al.43 In another study, a lower number of γδ T cells was seen in patients who developed chronic GVHD than in patients who did not develop chronic GVHD.44
In a larger study in patients with leukemia, who received a T-cell depleted bone marrow graft from partially HLA-mismatched donors, a significantly better disease-free survival (DSF) at 30 months posttransplant was observed in those patients in whom the percentage of γδ T cells exceeded 10% of the total lymphocytes.45 Most interestingly, there was no significant difference in the incidence of acute or chronic GVHD, suggesting an enhanced GVL effect in the absence of GVHD. In an extended 42-month follow-up study, these data were confirmed46 and a further 8-year follow-up study with additional patients (n = 153) showed a significantly better 5-year leukemia-free survival and overall survival for patients who recovered with an increased proportion of γδ T cells.47
A more recent study on the reconstitution of γδ T cells was performed with 102 pediatric patients with leukemia.48 In this single-center study at St. Jude Children’s Research Hospital, the number of γδ T cells was analyzed after allogeneic HCT of unmanipulated grafts from matched sibling, matched unrelated, or unrelated cord blood donor or of CD3+ T-cell depleted mobilized peripheral stem cell grafts from haploidentical donors. A significantly better event-free survival (EFS) and overall survival (OS) were observed in patients with elevated γδ T cells within a median follow-up of 2.7 years. Interestingly, a significantly lower incidence of infections, with no bacterial infection, was seen in the patients with high levels of γδ T cells, suggesting an important antibacterial49 and antiviral function of these cells.3 The anti-CMV response of Vδ2neg γδ T cells after allo-HCT especially seems of interest, because this subset expands upon CMV reactivation, and these CMV-induced γδ T cells are able to recognize and also lyse primary leukemic blasts.50 This may at least in part explain the favorable effect of CMV reactivation after HCT on the risk of relapse.51
In a manner similar to NK cells, γδ T cells express inhibitory HLA class I receptors. This might play a role in tumor immunity and autoimmunity,52,53 and it has been shown that Vγ9δ1 T cells isolated from the peripheral blood of an AML patient after HCT effectively lysed freshly isolated AML blasts and AML cell lines and that the inhibitory killer immunoglobuline-like receptor (KIR) CD158b negatively regulated the cytotoxic function of the γδ T cells.54 It is currently not clear, however, whether alloreactive and nonalloreactive γδ T cells based on their KIR expression exist and whether the donor KIR phenotype or genotype plays a role in allogeneic HCT in the context of γδ T cell recovery, as it has been described for alloreactive NK cells.55
Adoptive transfer of autologous and allogenic γδ T cells
Protocols for ex vivo–manipulations of γδ T cells
Various methods for the ex vivo expansion of γδ T cells for autologous and allogeneic adoptive transfer have been established. Combinations of interleukin 2 (IL-2) and IPPs or aminobisphosphonates (eg, zoledronate) are established protocols for their expansion from peripheral blood mononuclear cells (PBMCs).56 Incubation of PBMCs with zoledronate via the inhibition of the farnesyl pyrophosphate synthase leads to the accumulation of mevalonate metabolites, such as IPPs selectively in monocytes, which become APCs and stimulate Vγ9Vδ2 T cells. Therefore monocytes are required for γδ T cell expansion.57 In the absence of monocytes, IPPs or related phophoantigens are necessary. Besides IL-2, other cytokines that share the common cytokine receptor γ chain γc are investigated, and it has been shown that IL-15 combined with IPPs enhanced the proliferation of purified γδ T cells.58 In addition, the expanded γδ T cells had an activated phenotype, exerted increased cytotoxicity toward lymphoma and multiple myeloma target cells, and secreted high amounts of proinflammatory cytokines, comprising interferon-γ (IFN-γ) and tumor necrosis factor–α (TNF-α). Adding IL-15 to IL-2/zoledronate also boosted the expansion of γδ T cells from PBMCs.
Although most clinical trials of adoptive transfer of ex vivo expanded γδ T cells have been performed with autologous T cells and in solid tumors,59 only 1 study so far has been performed with patients with hematologic malignancies by using allogeneic γδ T cells. The successful adoptive transfer and in vivo expansion of haploidentical γδ T cells was reported by Wilhelm et al in a nontransplant setting.60 In this study, unstimulated leukapheresis products from haploidentical family donors were CD4- and CD8-depleted and the remaining γδ T cells infused into patients after lymphopenia-induced chemotherapy comprising fludarabine and cyclophosphamide. The median of infused γδ T cells was 2.17 × 106/kg (range, 0.9-3.84). After infusion, patients received zoledronate at day 0 and 1 × 106 IU/m2 IL-2 on day +1 through +6. A marked expansion of γδ T cells (mean, 68-fold) was observed, with a peak at day 8 and persistence up to 28 days.
Despite moderate results in hitherto performed clinical studies in solid malignancies,61 intratumoral γδ T-cell signatures emerged as the most significant favorable cancer-wide prognostic populations in 25 human cancers, including lymphoma and multiple myeloma, thus pointing to a key role of these cells in cancer immune surveillance.62
Adoptive transfer of γδ T cells after allo-HCT
Another method for the adoptive transfer of allogenic γδ T cells in transplant settings with a stem cell graft is the use of mobilized peripheral stem cells (PBSCs) depleted from T-cell receptor–αβ (TCRαβ) positive T lymphocytes using a clinical-grade technology first described by Chaleff et al.63 The purpose of this strategy is to conserve mature NK and γδ T cells, both of these lymphocyte subsets presumably being active against leukemia and viral infections but having a limited role in inducing GVHD. It was shown by Otto et al that stem cell mobilization of the donors with granulocyte colony-stimulating factor (G-CSF) does not impair the function of γδ T cells and that they retain strong tumoricidal activity and produce immunomodulatory cytokines, such as IFN-γ and TNF-α.64 Interestingly, in some of the G-CSF-mobilized donors, a high proportion of Vγ9Vδ1 T cells was seen. This subset was associated with effective killing of fresh AML blasts after allogeneic HCT,54 and more research is necessary to investigate the influence of G-CSF mobilization on γδ T cells and their subsets.
The TCRαβ depletion method was further extended by Schumm et al65 and was performed in 102 mobilized apheresis products. The median number of γδ T cells in the mobilized grafts prior to depletion was 345 × 106 (range, 16-2852) and 272 × 106 (range, 20-2339) after TCRαβ depletion, with a median recovery of γδ T cells of 83%. An improved immune recovery after TCRαβ-depleted stem cells from haploidentical donors in pediatric patients was reported by Lang et al.66 The median number of cotransplanted γδ T cells was 11 × 106/kg (range, 0.9-89.7). Recovery of donor-derived γδ T cells already started at day +7 posttransplant and preceded the reconstitution of αβ T cells. In the early posttransplant period, γδ T cells represented the majority of CD3+ lymphocytes. Similar results were reported in children with AML who received TCRαβ-depleted grafts from matched unrelated donors.67 Airoldi et al report a more detailed analysis of the γδ T-cell reconstitution after haploidentical transplantation of PBSCs that were depleted of αβ T cells.68 These authors analyzed the γδ T-cell reconstitution and their Vγ9Vδ2 and Vγ9Vδ1 subsets in 27 pediatric patients. Fifteen of the patients had acute leukemia. The median number of γδ T cells coinfused with the graft was 7.85 × 106/kg (range, 1.5-94.5). Phenotypic analysis of the reconstituting lymphocytes collected 2 to 4 weeks posttransplant also showed that the majority of the CD3+ T lymphocytes were of the γδ lineage (mean, 91.5% of the gated CD3+ T cells) ranging from 70% to 100%. As has been reported already by Lang et al,66 the γδ T- cell population decreased over time, and the percentage of the αβ T cells exceeded that of γδ T cells in most patients after 2 months posttransplant. Interestingly, a higher number of infused γδ T cells was significantly correlated with a later increase of αβ T cells. A more detailed analysis of the reconstituting γδ T-cell subsets mainly showed 3 subsets, namely Vδ1+, Vδ2+, and Vδ1negVδ2neg γδ T cells. One month posttransplant, γδ T cells were Vδ2+ (mean, 65%) and Vγ1+ (mean, 25%) and Vδ1negVδ2neg (9.6%). This composition was very similar to that of the γδ subsets present in the mobilized stem cell donors as well as in healthy individuals. The authors then compared the γδ T-cell recovery with pediatric patients who had received a CD34+-enriched haploidentical graft and found a significant difference. Three months posttransplant, the percentage of γδ T cells in the CD34+ cohort was significantly lower, and most cells were of the Vδ1 phenotype. In those patients who experienced CMV reactivation, a significant increase of the Vδ1 subset was observed. Incubation of the γδ T cells collected at various time points posttransplant with zoledronate and IL-2 led to an expansion of the Vγ9Vδ2 T cells. Although the expanded cells showed only a low cytotoxicity toward primary leukemia cells, the lysis capability was strongly augmented by sensitizing the leukemic target cells with zoledronate. This was shown for primary myeloid blasts, T-ALL, and B-cell precursor ALL blasts.
The same group then presented very interesting data on the clinical application of zoledronate to 43 pediatric patients with ALL (n = 30) and AML (n = 13), all of whom received a haploidentical TCRαβ-depleted PBSC graft.69 Zoledronate was given every 28 days, and most patients were treated at least twice. Treatment with zoledronate was associated with an increase of Vδ2 subsets and an increase of the cytotoxicity against primary leukemic blasts. Patients given 3 or more infusions of zoledronate had a better probability of survival than did those who received only 1 or 2 treatments (86% vs 54%, respectively; P = .008). It is noteworthy that all the reported studies on haploidentical transplantation of TCRαβ-depleted PBSC grafts were performed with no or only short-term pharmacological GVHD prophylaxes, which might favor a better immune recovery than those with transplantation of T-replete grafts with long-term posttransplant immunosuppression for GVHD prophylaxis.70 Whether the rapid expansion of γδ T cells early after transplantation can exclusively be explained by the expansion of the coinfused donor γδ T cells is yet unclear, because Ziegler et al have shown that a small population of CD4+Vδ1+ can transdifferentiate extrathymically directly into αβ T cells under inflammatory conditions.71 This small subset also expresses CD34 and is present in donor PBSCs and in TCRαβ-depleted grafts. Further research is necessary to investigate whether this subset contributes to the improved αβ T-cell recovery after transplantation of TCRαβ-depleted grafts.
Future perspectives
The fact that γδ T cells do not cause GVHD but can exert strong GVL effects and can mediate strong antiviral and antibacterial activity predisposes this cell population for cellular immunotherapeutic strategies in the context of allogeneic HCT.72 In vivo activation of γδ T cells posttransplant by the repetitive infusion of zoledronate69 has shown promising results in pediatric leukemia, and further clinical studies are warranted, also in combination with cytokines (eg, IL-2 and IL-15).
Because γδ T cells can be obtained and expanded in vivo and ex vivo from stem cell donors as well as from patients after lymphocyte recovery,60 various strategies for posttransplant adoptive transfer can be envisioned, depending on the purpose of the adoptive transfer. γδ T cell–enriched TCRαβ-depleted PBSC grafts have been used as an immunological booster to treat graft failure in 5 patients after transplantation of HLA-matched related and unrelated grafts.73 Besides NK cells, such donor lymphocyte infusions (DLIs) mainly comprise Vγ9Vδ2 T cells and lead to increased levels of white blood cells, platelets, granulocytes, or a combination of these 30 days after infusion. The number of infused γδ T cells ranged from 0.2 × 106/kg to 83.5 × 106/kg, and most important, no signs of GVHD were seen. A positive effect on the infectious complications was seen in 4 of the 5 patients. Therefore, future clinical studies using γδ T-cell DLIs in patients with graft failure, decreasing donor chimerism, or posttransplant infectious complications are warranted. It is currently not clear whether different subsets of γδ T cells would have different effects.
It has already been reported that CMV reactivations after allogeneic HCT led to an in vivo expansion of Vδ2neg γδ T cells, which then also cross-recognized leukemic blasts.50 Therefore, the adoptive transfer of Vδ2neg-donor γδ T cells could be an alternative to the use of CMV-specific αβ T cells,74 because this subset is also present in CMV-naïve cord bloods50 and in CMV negative donors. On the basis of the cross-recognition of CMV-induced Vδ2neg T cells of leukemic blasts, this subset would also be of interest as DLIs to prevent or treat leukemic relapses. In this context, a Vδ2neg Vδ1+ subset is of increasing interest because of its antimalignancies activity.75
Circulating Vδ1+ T cells have been associated with nonprogression of low-risk B–chronic lymphocytic leukemia (B-CLL)76 and showed effective antitumor responses against neuroblastoma target cell lines.77 Although some activating ligands are known, such as stress-induced self-antigens, other ligands are still to be identified. CD1d is widely recognized by Vδ1+ T cells by germ line residues spanning the complementarity-determining region (CDR) loop of Vδ1, and CD1d-bound α GalCer78 and sulfatide are separately recognized by non–germ line CDR3δ-encoded residues. These findings provide structural demonstration of MHC-like recognition of a self-lipid by γδ T cells and reveal the prevalence of lipid recognition by innate-like T-cell populations79 ; they may also be useful for manipulating the number of γδ T cells. CD1-bound α GalCer has already been investigated in clinical trials in patients with asymptomatic myeloma in context with invariant NKT cells80 and might also be considered in context with γδ T cells. Upon activation with IL-2, the small Vδ3 subset can also be expanded, recognize CD1d and lyse CD1d+ target cells, and release cytokines such as IL-17 and IFN-γ.81 Such strategies could also be envisioned posttransplant to exploit the antileukemia activity of Vδ subsets.
Although most clinical ex vivo expansion strategies expand Vγ9Vδ2 T cells, various small-grade procedures, but only 1 clinical-grade method for the selective ex vivo expansion of Vδ1 cells, have been described. The latter expands Vδ1+ cells up to 2000-fold and generates Th1 cytokine but not IL-17-producing, long-lived functional Vδ1+ T cells, which inhibited tumor growth and prevented dissemination of CLL xenografts.82 Siegers used mitogen-stimulated γδ T cells and IL-2 and IL-4.83 Upon injection into leukemia-bearing mice, Vδ1+ cells after having been expanded ex vivo for 16 to 21 days were still viable 5 weeks after injection. A 24 000-fold expansion was achieved with PBMCs that were stimulated with Concanavalin A and then depleted for αβ T cells. Lamb et al reported a 1200-fold expansion of Vδ1+ T cells from PBMC after depletion of αβ T cells and culture with irradiated leukemia feeder cells and low levels of IL-2.30 An additional approach to expand Vδ1+ and Vδ2+ T cells is the use of an anti-γδ TCR antibody.84 These expanded γδ T cells showed an effective antilymphoma cytotoxicity and might be more suitable for adoptive immunotherapy of lymphoid malignancies.
Besides ex vivo expansion, γδ T cells can be redirected against tumor targets using chimeric antigen receptors (CARs),85 and peripheral blood-derived Vγ9Vδ2 T cells were transduced with retroviral vectors encoding either disialoganglioside GD2- or CD19-specific CARs.86 These γδ CAR T cells could be expanded and showed antigen-specific IFN-γ secretion and cytotoxcity against GD2+ neuroblastoma cells and CD19+ leukemic blasts. Deniger et al electroporated PBMC for the transfer of sleeping-beauty transposase and a synthetic CD19-specific T-cell receptor encoding transposon to enforce expression of CD19-specific CARs. The CARpos γδ T cells were expanded on CD19+ artificial APCs and showed a polyclonal repertoire of endogenous γδ T-cell receptors and an enhanced killing of CD19+ leukemic blasts in comparison with CARneg γδ T cells. After adoptive transfer of the CARpos γδ T cells to CD19+ leukemia-bearing NSG mice, the tumor burden was significantly reduced in comparison with untreated mice.87 Another approach comprises the ex vivo expansion of cytokine-induced killer cells and Vγ9Vδ2 T cells for CAR T-cell therapy.88 PBMCs were expanded in vitro by using zoledronate, IFN-γ, IL-2, anti-CD3 antibody, and engineered K562 feeder cells. A nearly 20 000-fold expansion was achieved, with over 20% of the expanded cells being γδ T cells. The expanded cells were then modified to express CD19 CARs and showed enhanced cytotoxicity in vitro as well as in vivo in a xenograft model.88 γδ T cells can also be redirected against leukemia by introducing an exogenous αβ TCR of known antitumor specificity in γδ T cells.89,90 These cells retain the functionality of their original TCR and respond to stimuli via αβ and γδ TCR with rapid, γδ-like kinetics.91 Another approach is the transduction of PAg-reactive Vγ9Vδ2 TCR into αβ T cells to redirect them to cancer cells. The concomitant downregulation of the endogenous αβ TCRs might abrogate alloreactive responses, and such effector cells might also play a role in the allogeneic HCT setting.92 This strategy not only overcomes diversity of different affinities of γδ TCRs but also generates appropriate help through CD4-engineered cells.92
Another interesting approach is the use of bispecific T-cell engager (BiTE) antibodies, which are engineered by combining 2 single chain (sc) Fv domains of 2 different antibodies. One scFv binds to effector cells, and the other scFv binds to a tumor-associated target. In ALL, the CD3-CD19 BiTE blinatumomab has shown impressive clinical efficacy in the treatment of ALL with manifest disease in children93,94 as well as in adults.95 It also showed impressive efficacy in the treatment of ALL patients with minimal residual disease.96 Moreover, BiTEs are currently also developed for the treatment of AML and solid tumors.97 A similar strategy has been described for γδ T cells,98 and a Her2 × Vγ9 BiTE has been generated. The construct triggered target-cell lysis by γδ T cells with freshly isolated PBMC or after short-term activation. While this bispecific antibody did not induce γδ T-cell proliferation and expansion, it might be more suitable for tumor cell killing than for γδ T cells activated with PAgs, mainly because in contrast to PAgs activation, the Her2 × Vγ9 BiTE did not trigger activation-induced cell death, which might hamper the antitumor efficacy of PAgs-activated γδ T cells. On the basis of the lack of alloreactivity of γδ T cells, such bispecific antibodies (eg, anti-CD19/anti-Vγ9) could also be envisioned for the treatment of CD19+ ALL or other leukemias in the context of allogeneic HCT.
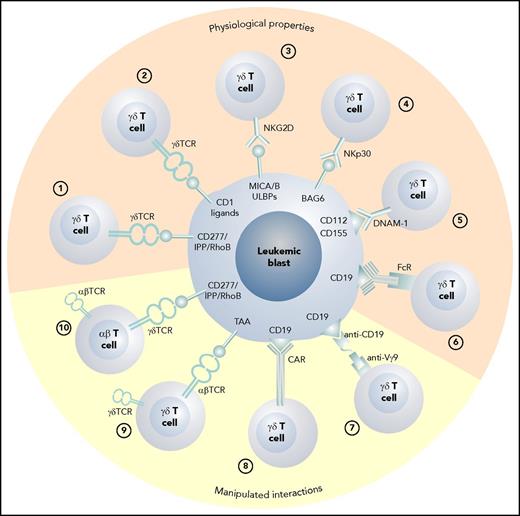
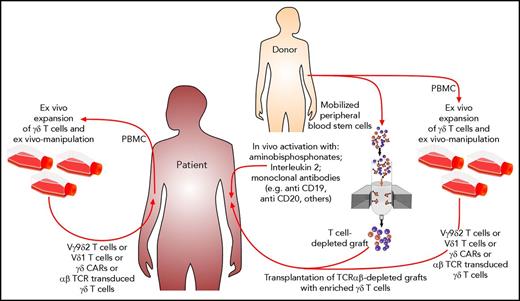
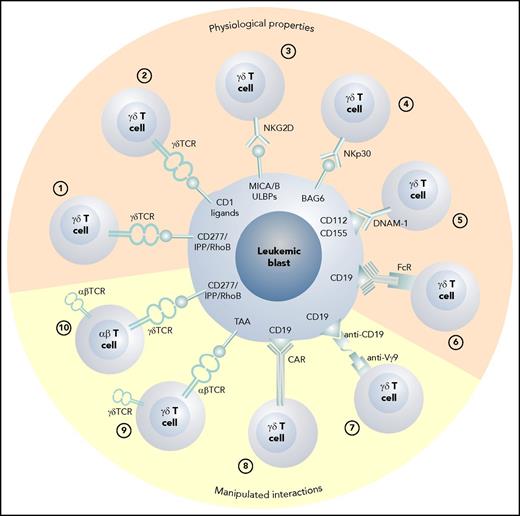
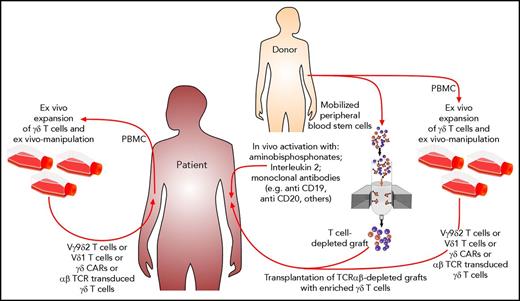
The various possible approaches to exploiting γδ T cells as effector cells against leukemic blasts are depicted in Figure 1. In Figure 2, possible scenarios show how γδ T cells can be activated directly in vivo and how patient- or donor-derived γδ T cells can be expanded, manipulated, and adoptively transferred into the patient after allogeneic HCT.
The various physiologic and manipulated antileukemic cytotoxic mechanisms of γδ T cells. (1) Vγ9Vδ2 T cells recognize blasts via the CD277/IPP/rhoB axis,34 and (2) CD1 ligands are recognized by Vδ1 γδ T cells.78 (3) Stress-induced self-ligands (MICA/B, ULBPs) are recognized by NKG2D15 ; (4) human leukocyte B–associated transcript 3 is recognized by natural cytotoxicity receptors NKp3018 ; (5) nectin-2 (CD112) and poliovirus receptor (CD155) are the ligands for DNAM-119,20 ; and (6) therapeutic monoclonal antibodies can induce antibody-dependent cellular cytotoxicity via the Fc receptor III.39 (7) Bispecific antibodies can activate γδ T cells via anti-Vγ9 single chain Fv,98 and (8) γδ T cells can be redirected using CARs86 or (9) through the transduction of an exogenous αβ TCR directed against a tumor-associated antigen.89 (10) The transfer of a Vγ9Vδ2 TCR recognizing blasts via the CD277/IPP/rhoB axis with optimum affinity for the target into αβ T cells also confers appropriate help through engineered CD4 T cells.92 FcR, Fc receptor III; TAA, tumor-associated antigen.
The various physiologic and manipulated antileukemic cytotoxic mechanisms of γδ T cells. (1) Vγ9Vδ2 T cells recognize blasts via the CD277/IPP/rhoB axis,34 and (2) CD1 ligands are recognized by Vδ1 γδ T cells.78 (3) Stress-induced self-ligands (MICA/B, ULBPs) are recognized by NKG2D15 ; (4) human leukocyte B–associated transcript 3 is recognized by natural cytotoxicity receptors NKp3018 ; (5) nectin-2 (CD112) and poliovirus receptor (CD155) are the ligands for DNAM-119,20 ; and (6) therapeutic monoclonal antibodies can induce antibody-dependent cellular cytotoxicity via the Fc receptor III.39 (7) Bispecific antibodies can activate γδ T cells via anti-Vγ9 single chain Fv,98 and (8) γδ T cells can be redirected using CARs86 or (9) through the transduction of an exogenous αβ TCR directed against a tumor-associated antigen.89 (10) The transfer of a Vγ9Vδ2 TCR recognizing blasts via the CD277/IPP/rhoB axis with optimum affinity for the target into αβ T cells also confers appropriate help through engineered CD4 T cells.92 FcR, Fc receptor III; TAA, tumor-associated antigen.
Possible scenarios for how antitumor function of nonalloreactive γδ T cells could be exploited after HCT. Aminobisphosphonates in combination with IL-2 or the application of therapeutic monoclonal antibodies can activate γδ T cells in vivo. By using TCRαβ-depleted grafts, large numbers of γδ T cells are coinfused. γδ T cells and their subsets either from patients or from their donors can be expanded ex vivo and manipulated prior to adoptive transfer into the patients.
Possible scenarios for how antitumor function of nonalloreactive γδ T cells could be exploited after HCT. Aminobisphosphonates in combination with IL-2 or the application of therapeutic monoclonal antibodies can activate γδ T cells in vivo. By using TCRαβ-depleted grafts, large numbers of γδ T cells are coinfused. γδ T cells and their subsets either from patients or from their donors can be expanded ex vivo and manipulated prior to adoptive transfer into the patients.
Conclusions
Over the years, γδ T cells have been established as important effector cells for immunotherapeutic strategies in hematologic malignancies in the context of allogeneic stem cell transplantation (allo-SCT). Many clinical trials exploiting in vivo activation or ex vivo expansion of γδ T cells have been or are currently being performed,99 and a meta-analysis of γδ T-cell-based tumor immunotherapy in various tumor entities showed their efficacy and safety.100 Preliminary data on the antileukemic efficacy in the allogeneic HCT setting are promising, and more clinical studies in hematological malignancies are warranted. The introduction of the clinical-grade TCRαβ depletion mainly in the haploidentical setting and the coinfusion of large numbers of γδ T cells together with the stem cell grafts provided valuable insights into the antileukemic and anti-infectious effects of γδ T cells in allogeneic HCT.
However, it is still not clear which subset of γδ T cells is more effective, and it might well be that different subsets are necessary for different purposes. Accumulating evidence suggests that the Vδ1 subset will play an important role in the prevention or treatment of posttransplant relapses of ALL and AML and that it might become feasible in the future to separate a potent GVL effect from GVHD.101
Because γδ T cells do not exert dysregulated alloreactivity (but rather dampen excessive, unwanted αβ T-cell responses) while simultaneously showing potent antileukemic activity, approaches to in vivo activate γδ T cells directly or expand them ex vivo, either from the stem cell donor or from the patient posttransplant, might offer promising therapeutic strategies for preventing or treating relapse after HCT. However, more basic and clinical research will be necessary to fully exploit the antileukemic effect in the setting of allogeneic transplantation.
Acknowledgments
The authors thank Peter Weber for the graphic art.
This work was supported by grants from the Jürgen Manchot Foundation (K.S.), and by grants from the Stiftung für Krebskranke Kinder Tübingen e.V., the Jose-Carreras Leukemia Foundation, and the Stefan-Morsch-Stiftung (R.H.).
Authorship
Contribution: R.H. and K.S. wrote the review.
Conflict-of-interest disclosure: R.H. is a copatent holder (Germany) of the T-cell receptor αβ depletion technology, and has received speaker honoraria from Miltenyibiotec. K.S. declares no competing financial interests.
Correspondence: Rupert Handgretinger, Department of Pediatric Hematology and Oncology, University Children’s Hospital, Hoppe-Seyler Str 1, D-72076 Tuebingen, Germany; e-mail: rupert.handgretinger@med.uni-tuebingen.de.