In this issue of Blood, Shapiro et al describe the first-in-class experience of infusing plasma-derived Glu-plasminogen to 14 patients with congenital deficiency of plasminogen as part of an ongoing, phase 2/3, open-label clinical trial, reporting encouraging results.1 Congenital plasminogen deficiency is caused by homozygous or compound-heterozygous mutations in the plasminogen (PLG) gene, located on chromosome 6q26. It is, in the words of the authors, “ultra-rare,” predicted to affect ∼1 to 2 per million people.2 Plasminogen is activated to plasmin and is critical for intravascular and extravascular fibrinolysis. Other functions include wound healing, cell migration, tissue remodeling, angiogenesis, and embryogenesis. Glu-plasminogen has a glutamic acid residue at its N-terminus, as opposed to Lys-plasminogen, which has a lysine. Glu-plasminogen is the predominant form found in circulation and has a half-life of 2 to 2.5 days, whereas the half-life of Lys-plasminogen is 0.8 days.3
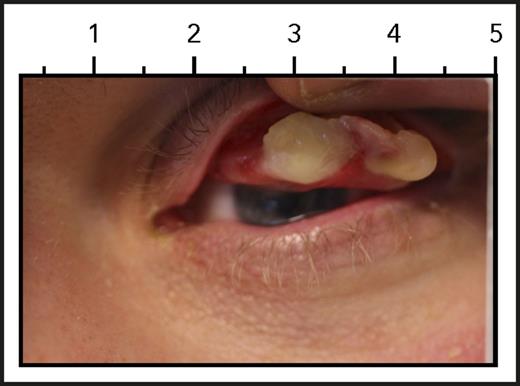
Ligneous conjunctiva. See Figure 3A in the article by Shapiro et al that begins on page 1301.
Ligneous conjunctiva. See Figure 3A in the article by Shapiro et al that begins on page 1301.
Patients lacking plasminogen suffer from abnormal growth of fibrin-rich, woodlike (“ligneous”) pseudomembranes on mucous membranes, such as conjunctiva, gingiva, airways, and the vaginal tract, typically within the first year of life.4 Ligneous conjunctiva is the pathognomonic manifestation of congenital plasminogen deficiency (see figure). If left untreated, these woody growths over time severely affect quality of life and eventually lead to organ dysfunction, including blindness.
Historically, local therapy was the only available treatment. Topical eye drops containing plasminogen only treated conjunctival lesions, and surgery to remove the pseudomembranes predisposed to scar tissue and regrowth of lesions. The first publication reporting the successful use of IV infusions of unlicensed Lys-plasminogen to treat widespread lesions in a toddler with homozygous plasminogen deficiency came out in 1998.5 Because of the shorter half-life of Lys-plasminogen, this toddler was treated with a continuous infusion initially, followed by daily infusions. In comparison, based on individual pharmacokinetic studies, Glu-plasminogen used by Shapiro et al allowed for infusions every 2 to 4 days in these 14 patients. All evaluable lesions in these patients improved or resolved within the 12-week study window, and most improved after only 4 weeks of treatment.
Adverse events were largely limited to headache, nasopharyngitis, and gastrointestinal distress (abdominal pain, nausea, and/or diarrhea). Reassuringly, bleeding complications appear minimal, with only 2 patients each reporting epistaxis, hematuria, or dysmenorrhea during their treatment with Glu-plasminogen.
An astute reader or a scholar of congenital plasminogen deficiency will notice that thromboembolic disease is not a sequela of congenital plasminogen deficiency and that the disease manifests solely with extravascular deposition of fibrin. Despite initial reports implicating heterozygous hypoplasminogenemia as a risk factor for thromboembolic disease, more recent epidemiologic studies have not found an increase in thrombosis compared with those with normal levels of plasminogen.2 Furthermore, thrombosis has not been described in patients with homozygous or compound-heterozygous plasminogen deficiency.2 This suggests that other pathways, such as those involving cathepsins, elastases, or matrix metalloproteinases, can cleave intravascular, but not extravascular, fibrin and highlights how much more we need to learn about fibrinolysis.
Undoubtedly, many hematologists (cardiologists, stroke-ologists, and vascular surgeons too) are already daydreaming about all the indications that a widely available, licensed Glu-plasminogen product may serve for a myriad of acquired conditions. However, let us remember that (1) this is still an ongoing clinical trial that has shown preliminary efficacy in improving extravascular fibrin deposition and (2) other hemostatic products marketed for congenital conditions that were used off-label for acquired disease have demonstrated less than stellar outcomes.6,7 Instead, let us celebrate that a potential treatment is one step closer to helping patients with this devastating orphan disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.