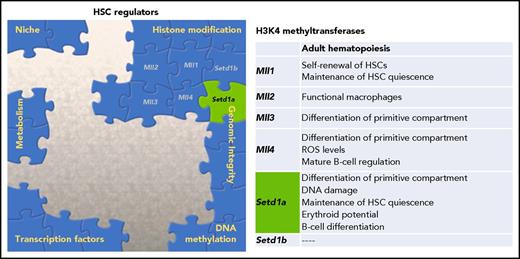
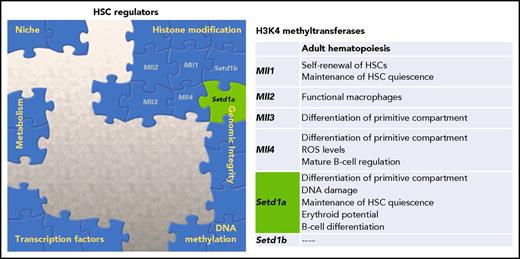
In this issue of Blood, Arndt et al demonstrate the unique role of the histone methyltransferase Setd1a in regulating hematopoietic stem cells (HSCs) and provide a connection between 2 drivers of aberrant stem cell function, epigenetic dysregulation and DNA damage.1
Multiple factors contribute to the overall regulation of HSC potential. One family of histone modifiers, H3K4 methyltransferases, has been characterized, and Arndt et al provide novel data supporting a dual role of Setd1a in both epigenetic regulation and genomic integrity. ROS, reactive oxygen species.
Multiple factors contribute to the overall regulation of HSC potential. One family of histone modifiers, H3K4 methyltransferases, has been characterized, and Arndt et al provide novel data supporting a dual role of Setd1a in both epigenetic regulation and genomic integrity. ROS, reactive oxygen species.
HSCs are responsible for maintenance of the hematopoietic compartment and for the restoration of the system during conditions of stress or loss of homeostasis. Regulation of stem cell fidelity is considered paramount, as these self-renewing cells ensure the capacity for life-long hematopoietic function. To safeguard HSCs, cells are imbued with cytoprotective features to mitigate alterations to their functional potential and are regulated by the orchestration of both intrinsic and extrinsic signals. As we continue to piece together the molecular guardians of HSC potential (see figure), it is becoming more evident that this complex network is interconnected and that key regulators may play multiple roles in this maintenance.
Epigenetic regulation of HSC function and fate decisions has been a burgeoning topic in recent years. This literature has established that histone modifications, DNA methylation, and ncRNA are cornerstones of the HSC regulatory network.2,3 The role the MLL/SET family of histone methyltransferases plays in hematopoiesis has been of particular interest because of the association of leukemias with chromosomal rearrangements of MLL1. The MLL/SET family is composed of 6 members (Mll1/KMT2A, MLL2/KMT2B, MLL3/KMT2C, MLL4/KMT2D, SETD1A/ KMT2F, and SETD1B/KMT2G), and they methylate lysine 4 of histone H3 (H3K4). Previous work has demonstrated using constitutive knockouts of the MLL/SET family that loss of these methyltransferases results in embryonic death.4 Although all MLL/SET proteins are critical for development, each factor appears to have specific roles in regulating adult hematopoiesis and stem cell function,1,4 including playing a role in white blood cell output, B-cell development, and erythropoiesis (see figure).5,6 Waskow’s group uses a series of conditional knockout mice to elucidate the function of Setd1a in the primitive hematopoietic compartment.1 Global loss of Setd1a led to significant reduction in peripheral blood reconstitution and early lethality, suggesting dysfunctional hematopoiesis causing death. To address the cell autonomous nature of this loss, competitive bone marrow transplants were performed, and again donor cells lacking Setd1a expression failed to contribute to peripheral blood. The authors further show a significant decrease in erythroid progenitors (common myeloid progenitors and megakaryocyte erythroid progenitors), confirming the role of Setd1a in erythropoiesis.6 However, in contrast to the loss of more differentiated cells, the overall donor-derived Lineage− Sca-1+ cKit+ (LSK) compartment from the Setd1a knockouts was expanded, largely because of increased numbers of multipotent progenitors. To more definitely examine the effect on the stem cells, Arndt et al challenged the primitive compartment lacking Setd1a expression with proliferation, either by secondary transplants or 5-fluorouracil stress, and again recapitulated the significant loss of differentiation potential.
The authors demonstrate that many of the aberrant HSC functions were only manifest in environments of proliferative challenge.1 These data may contribute to the discussion of the role HSCs play in steady-state hematopoiesis. Studies using transposon-tracking bar codes have supported the hypothesis that HSCs have minimal contribution to steady-state hematopoiesis and the drivers during adulthood are largely the progenitors cells.7,8 Yet, the debate has been reopened as Sawai et al recently provided evidence supporting a larger contribution from HSCs in adult mice.9 Loss of Setd1a in Scl-expressing cells (largely the primitive compartment) led to only mild perturbations in the blood over a 6-month follow-up,1 perhaps lending support to the lesser role of HSCs during steady-state hematopoiesis.
To investigate the role of Setd1a in the cell cycle and cell division, the authors demonstrate a decreased number of quiescent cells in the primitive compartment lacking Setd1a expression. Surprisingly, elevated cycling activity was not correlated to an increase in ROS.1 In fact, ROS levels were significantly decreased in the Setd1a-knockout cells. However, the authors show a significant increase in global DNA damage in these knockout cells and a significant decrease in expression of DNA damage repair pathway genes in LSK cells. This is interesting as the Setd1a knockout cells are less quiescent, which might have been correlated with increased repair and response pathways as many of the DNA damage repair and response genes are intimately tied to the cell cycle. Arndt et al correlate reduced expression of DNA repair genes to reduced levels of H3K4me3 at the promoter of several genes in DNA repair pathways in the Setd1a knockout suggesting a direct connection between DNA repair and this histone methyltransferase. The methyltransferase activity of Setd1a appears to be essential for the function, whereas other MLL/SET family members can also regulate hematopoiesis in an indirect fashion,4 supporting diversity of their modes of action. Further, many of the phenotypes of this Setd1a knockout in the primitive compartment mirror the effects of the conditional knockout of Dpy30, a core subunit for the MLL family of H3K4 methyltransferases. Both knockouts show an expansion of the LSK compartment, loss of differentiation potential, and ROS decrease10 supporting histone methyltransferase activity as playing a driving role in the regulation.
Arndt et al have added an important piece to the “HSC regulation puzzle” that also highlights the importance of continuing to establish connections between regulators of HSCs (see figure). Waskow’s group also demonstrates differential results depending if experiments were performed in vivo or in vitro.1 Although not uncommon, it again suggests regulation by the niche needs to be factored into this overall regulation puzzle. Given the interesting decrease in ROS in these cycling cells, it will be also be relevant to examine the connection that Setd1a may play in HSC metabolism. Understanding the network of regulation controlling the HSC compartment is opening new means to examine aging and diseases of the hematopoietic system, which are associated with loss of epigenetic regulation, accumulation of DNA damage, and environmental alterations. It will be interesting to expand these studies characterizing regulators, such as Setd1a, that simultaneously affect multiple facets of stem cell regulation, in normal hematopoiesis and in settings of dysfunction.
Conflict-of-interest disclosure: The author declares no competing financial interests.