Key Points
100% response rate (92% CR) in 26 patients treated with frontline BV+CHP. After ∼5 years, 50% remained in CR (PFS 37.8+ to 66.0+ months).
18 of 19 patients (95%) with treatment-emergent PN reported resolution or improvement in symptoms; 9 had resolution of all PN events.
Abstract
This phase 1 study evaluated frontline brentuximab vedotin in combination with cyclophosphamide, doxorubicin, and prednisone (BV+CHP; 6 cycles, then up to 10 cycles of brentuximab vedotin monotherapy) in 26 patients with CD30+ peripheral T-cell lymphoma, including 19 with systemic anaplastic large cell lymphoma. All patients (100%) achieved an objective response, with a complete remission (CR) rate of 92%; none received a consolidative stem cell transplant. After a median observation period of 59.6 months (range, 4.6-66.0) from first dose, neither the median progression-free survival (PFS) nor the median overall survival (OS) was reached. No progression or death was observed beyond 35 months. The estimated 5-year PFS and OS rates were 52% and 80%, respectively. Eighteen of 19 patients (95%) with treatment-emergent peripheral neuropathy (PN) reported resolution or improvement of symptoms. Thirteen patients (50%) remained in remission at the end of the study, with PFS ranging from 37.8+ to 66.0+ months. Eight of these 13 patients received the maximum 16 cycles of study treatment. These final results demonstrate durable remissions in 50% of patients treated with frontline BV+CHP, suggesting a potentially curative treatment option for some patients. This trial was registered at www.clinicaltrials.gov as #NCT01309789.
Introduction
Peripheral T-cell lymphomas (PTCLs) encompass about 10% to 15% of aggressive non-Hodgkin lymphomas. Clinical outcomes for treatment-naïve patients vary extensively depending on PTCL subtype and therapeutic intervention. Although complete remission (CR) rates of approximately 30% to 70% following frontline anthracycline-based multiagent therapy have been reported for patients with anaplastic lymphoma kinase (ALK)-negative systemic anaplastic large cell lymphoma (ALCL) and other non-ALCL diagnoses, 5-year progression-free survival (PFS), and overall survival (OS) rates range from <5% to approximately 40% and from <10% to approximately 50%, respectively.1-5 Consolidation with autologous stem cell transplant improved 5-year PFS by 21% in 1 study and OS by 22% to 33%, resulting in 5-year PFS and OS rates ranging from 38% to 61% and from 47% to 70%, respectively.1,6
CD30 is expressed on many PTCL tumor cells including systemic ALCL, where it has high and uniform expression. The CD30-directed antibody–drug conjugate brentuximab vedotin has shown substantial activity as monotherapy across subtypes of relapsed or refractory PTCL.7,8
Our trial evaluated brentuximab vedotin administered in combination with cyclophosphamide, doxorubicin, and prednisone (BV+CHP) in 26 treatment-naïve patients with CD30-expressing PTCL. As previously reported, the safety profile was manageable and all patients achieved an objective response, including 23 with CR.9 One additional patient with partial remission subsequently converted to CR during brentuximab vedotin monotherapy, for a final CR rate of 92%. All 7 patients with non-ALCL diagnoses achieved CR.
This report summarizes durability of response and OS after approximately 5 years of follow-up, characterizes the patients in long-term follow-up who remained in remission with no additional therapy (n = 13), and describes the resolution of peripheral neuropathy (PN) in patients treated with BV+CHP.
Study design
The study design and methodology have been previously described.9 Combination therapy consisted of intravenous brentuximab vedotin, 1.8 mg/kg, with CHP (standard dose cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP] without vincristine) once every 3 weeks for 6 cycles. After 6 cycles, patients with objective response could receive up to 10 cycles of brentuximab vedotin monotherapy. Disease response was assessed by the investigators using the Revised Response Criteria for Malignant Lymphoma.10 Peripheral neuropathy was analyzed using a Standardized Medical Dictionary for Regulatory Activities Query; severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Before initiation, the study was approved by each site’s institutional review board, and written informed consent was obtained in accord with the Declaration of Helsinki.
We also evaluated brentuximab vedotin administered in sequence with CHOP. Despite a manageable safety profile, enrollment was terminated after patients who initially responded to brentuximab vedotin experienced disease progression while receiving CHOP.9 Efficacy outcomes are summarized in supplemental Table 1 (available on the Blood Web site).
Results and discussion
Combination BV+CHP was evaluated in 26 patients with CD30+ PTCL: 19 patients had systemic ALCL (16 ALK−, 3 ALK+) and 7 had other CD30+ PTCL (2 each adult T-cell leukemia/lymphoma, angioimmunoblastic T-cell lymphoma, and PTCL-not otherwise specified; 1 enteropathy-associated T-cell lymphoma). A median of 13 cycles (range, 3-16) of brentuximab vedotin was administered. Brentuximab vedotin dose reductions did not appear to affect survival (supplemental Table 2).
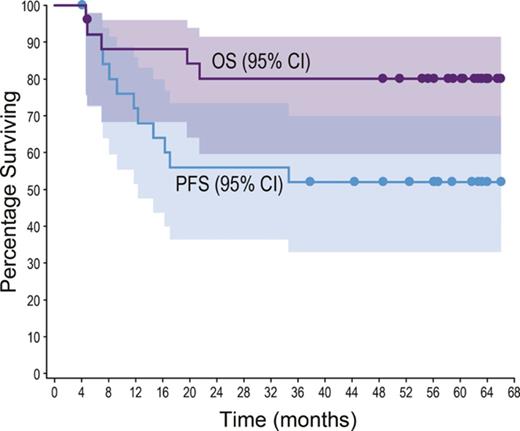
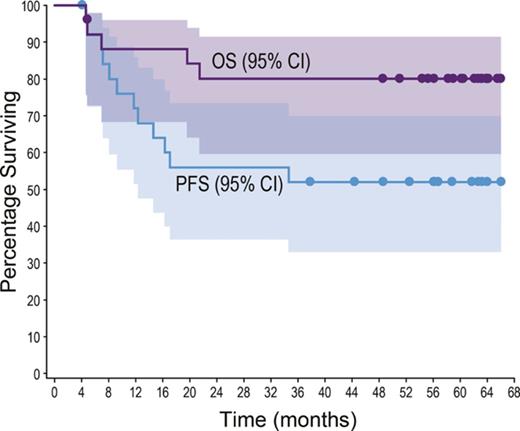
All patients achieved an objective response (24 CR, 2 partial remission). With a median observation time of 59.6 months (range, 4.6-66.0), 12 patients (46%; 10 ALCL, 2 non-ALCL) had experienced progressive disease or death. No progression or death was observed beyond 35 months (supplemental Table 3; supplemental Figure 1). The median PFS was not reached (Figure 1A); individual PFS ranged from 4.1+ to 66.0+ months. The estimated 5-year PFS rate was 52% (95% confidence interval [CI] 31, 69).
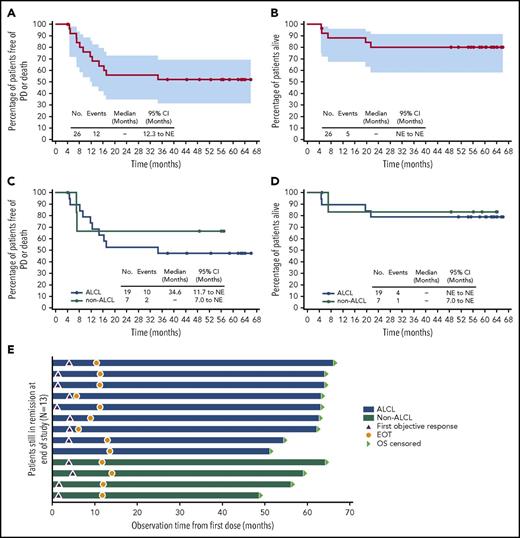
Five-year durability results. PFS and OS were analyzed using Kaplan-Meier methodology and are shown for the overall population (A-B) and by disease subtype (ALCL vs non-ALCL; C-D). All censored patients are indicated by dots on the Kaplan-Meier curves. One patient in remission (angioimmunoblastic T-cell lymphoma) did not enter long-term follow-up, withdrew consent, and was censored after 4.1 months. (A–B) Shading indicates the 95% confidence bounds. (E) Observation time for the subset of 13 patients in long-term follow-up remaining in remission with no subsequent anticancer therapy through end of study; all 13 achieved CR. Shading indicates disease subtype (ALCL vs non-ALCL). EOT, end of treatment; NE, not estimable; PD, progressive disease.
Five-year durability results. PFS and OS were analyzed using Kaplan-Meier methodology and are shown for the overall population (A-B) and by disease subtype (ALCL vs non-ALCL; C-D). All censored patients are indicated by dots on the Kaplan-Meier curves. One patient in remission (angioimmunoblastic T-cell lymphoma) did not enter long-term follow-up, withdrew consent, and was censored after 4.1 months. (A–B) Shading indicates the 95% confidence bounds. (E) Observation time for the subset of 13 patients in long-term follow-up remaining in remission with no subsequent anticancer therapy through end of study; all 13 achieved CR. Shading indicates disease subtype (ALCL vs non-ALCL). EOT, end of treatment; NE, not estimable; PD, progressive disease.
When analyzed by disease subtype (Figure 1C), the median PFS was 34.6 months (95% CI, 11.7-not estimable) for ALCL patients and was not reached for non-ALCL patients. The estimated 5-year PFS was 47% (95% CI, 24-67) for ALCL patients and could not be estimated for non-ALCL patients.
Five patients (19%; 4 ALK−ALCL, 1 enteropathy-associated T-cell lymphoma) died during long-term follow-up. Four deaths were disease related, and 1 patient died of respiratory failure (disease relatedness unknown). The median OS was not reached (Figure 1B); individual OS ranged from 4.6 to 66.0+ months. The estimated 5-year OS rate was 80% (95% CI, 59-91).
When analyzed by disease subtype (Figure 1D), the median OS was not reached for either subgroup of patients. The estimated 5-year OS was 79% (95% CI, 53-92) for ALCL patients and 83% (95% CI, 27-97) for non-ALCL patients. Among ALCL patients, outcomes for ALK+ patients appeared more favorable than those for ALK− patients (supplemental Table 4).
No patient received posttreatment consolidative stem cell transplant, which was allowed per protocol. After disease progression, 8 patients (31%) received subsequent therapy, including 3 with stem cell transplants. Initial postprogression therapies included single-agent brentuximab vedotin (n = 4; 50%).
After ∼5 years of follow-up, 13 patients (50%) remained in long-term follow-up and in remission without any new anticancer therapy. Nine of these patients had systemic ALCL, including all 3 ALK+ patients, and 4 patients had other diagnoses. All 13 patients achieved CR. Censored PFS values ranged from 37.8+ to 66.0+ months (Figure 1E). Individual characteristics of these 13 patients are provided in supplemental Table 5.
Baseline characteristics were generally similar for the patients remaining in remission compared with the other patients (Table 1). Collectively, disease stage at diagnosis tended to be earlier and baseline International Prognostic Index score tended to be lower for the patients remaining in remission. Patients remaining in remission received a median of 16 treatment cycles (range, 6-16) compared with 10 (range, 3-16) for the other patients.
Peripheral neuropathy is an adverse event associated with accumulated exposure to brentuximab vedotin. Nineteen patients (73%), including 12 remaining in remission, experienced treatment-emergent PN, predominantly sensory, that was a maximum of grade 1 or 2 for 17 patients and grade 3 for 2 patients. The median time to onset was 11.0 weeks (range, 0-34) for any grade of PN, 27.0 weeks (range, 9-51) for grade 2 (n = 13), and 34.1 weeks (range, 31-37) for grade 3 (n = 2).
Eighteen patients (95%) had resolution or improvement (by at least 1 grade) in PN symptoms, including 9 with resolution of all events. The median times to resolution and improvement were 4.2 and 2.6 months, respectively. Ten patients (53%) had ongoing PN at last follow-up, grade 2 for 1 patient who had no improvement during the study and grade 1 for 9 patients, including both patients with grade 3 events, 6 remaining in remission, and 1 other patient.
In summary, among 26 CD30+ PTCL patients treated with BV+CHP, the objective response rate was 100% and 92% achieved CR. After approximately 5 years, 50% remained in remission with no subsequent anticancer therapy. The median OS was not reached, and the estimated 5-year OS was 80%. Although 5-year PFS rates as high as 61% have been reported for PTCL, the safety of BV+CHP compares favorably to frontline anthracycline-based therapies followed by consolidative stem cell transplant.6,9 Given the high level of durable responses to this regimen and favorable safety profile, a randomized phase 3 clinical trial (www.clinicaltrials.gov # NCT01777152) compared frontline BV+CHP to CHOP for PTCL. Enrollment is complete and results are anticipated shortly.
The online version of this article contains a data supplement.
Presented in part at the Second European Society for Medical Oncology Congress, Madrid, Spain, 29 September 2014; the 57th, 58th, and 59th annual meetings of the American Society of Hematology (Orlando, FL, 5 December 2015; San Diego, CA, 4 December 2016; Atlanta, GA, 10 December 2017); and the 9th Annual T-cell Lymphoma Forum, San Francisco, CA, 27 January 2017.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Roberta Connelly for medical writing assistance and Teresa McClendon for statistical guidance, both under the sponsorship of Seattle Genetics, Inc.
This research was funded by Seattle Genetics, Inc., through the joint financial support of Seattle Genetics, Inc., and Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceuticals Limited. This work also was supported at Memorial Sloan-Kettering Cancer Center by a core facility grant from the National Institutes of Health National Cancer Institute (P30 CA008748).
Authorship
Contribution: M.A.F., S.M.H., B.P., R.W.C., D.A.K., and A.R.S. contributed to the concept and design of the study; M.A.F., S.M.H., A.F.-T., N.L.B., R.H.A., B.P., R.W.C., A.D., T.I., and A.R.S. contributed to the acquisition of the data; M.A.F. and H.R. wrote the manuscript; S.M.H., A.F.-T., N.L.B., R.H.A., B.P., R.W.C., A.D., T.I., M.U., S.-Y.L., D.A.K., and A.R.S. critically reviewed the manuscript; and all authors contributed to the analysis and interpretation of the data and approved the final version of the manuscript.
Conflict-of-interest disclosure: H.R., D.A.K. (at the time the work was done), and M.U. (at the time the work was done) are employees of and have equity ownership in Seattle Genetics, Inc. S.Y.-L. is an employee of and has equity ownership in Takeda Pharmaceuticals Limited. M.A.F. received research funding from Bristol-Myers Squibb, Merck & Co, Seattle Genetics, and Takeda; served on an advisory board for Seattle Genetics; received honoraria from Bristol-Myers Squibb, Merck & Co, Seattle Genetics, Takeda; served as a consultant for Bristol-Myers Squibb, Merck & Co, Seattle Genetics; and received travel expenses from Bristol-Myers Squibb, Merck & Co, Seattle Genetics, and Takeda. S.M.H. received research funding from ADCT Therapeutics, Aileron Therapeutics, Celgene, Forty Seven, Infinity/Verastem, Kyowa-Hakka-Kirin, Millennium/Takeda, Seattle Genetics, and Spectrum Pharmaceuticals and served as a consultant for Bristol-Myers Squibb, Celgene, Forty Seven, Infinity/Verastem, Kyowa-Hakka-Kirin, Millenium/Takeda, Mundipharma, and Seattle Genetics. A.F.-T. received research funding from and served on a speakers bureau for Seattle Genetics. N.L.B. received research funding from Affimed, Astra Zeneca, Bristol-Myers Squibb, Celgene, Forty Seven, Genentech, Gilead, ImaginAB, Immune Design, Janssen, KITE, Merck & Co, Millennium, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics and served on advisory boards for KITE, Pfizer, and Seattle Genetics. R.H.A. received research funding from Agensys, Bristol-Myers Squibb, Celgene, Forty Seven, Inc., Genentech/Roche, Infinity, Janssen Pharmaceutical, Kura, Merck, Millennium, Pharmacyclics, Regeneron, Seattle Genetics, and Teva Pharmaceuticals and served as a consultant for Astra Zeneca, Autolus, Bayer Healthcare Pharmaceuticals, Cell Medica, Seattle Genetics. B.P. received research funding from, served as a consultant for Seattle Genetics, and received honoraria and travel expenses from Seattle Genetics and Takeda Pharmaceuticals. R.W.C. received research funding from Bristol-Myers Squibb, Pharmacyclics, and Seattle Genetics; served as a consultant for Bristol-Myers Squibb, Genentech, Merck & Co, Pfizer, Pharmacyclics, and Seattle Genetics; and served on a speakers bureau for Genentech, Merck, and Seattle Genetics. A.D. received research funding from Acerta, Bayer, Celgene, Gilead, GSK, Janssen, Karyopharma, Pfizer, Roche, and Takeda; served on advisory boards for Celgene, CTI, Gilead, Karyopharma, Mundipharma, Roche, and Takeda; received honoraria from Celgene, CTI, Gilead, Janssen, Mundipharma, Pfizer, Roche, and Takeda; received travel expenses from CTI and Mundipharma and Takeda; and served as associate clinical director for the Southampton Clinical Trials Unit. T.I. received research funding from Seattle Genetics and honoraria from Takeda. D.A.K. has a patent (without royalties) with Seattle Genetics. A.R.S. received research funding from Actelion, Aileron, Bristol-Myers Squibb, Kyowa, Millennium/Takeda, Novartis, Pfizer, Seattle Genetics, and SPECTRUM and served as a consultant for SPECTRUM.
Correspondence: Michelle A. Fanale, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit No. 429, Houston, TX 77030; e-mail: mfanale@mdanderson.org.