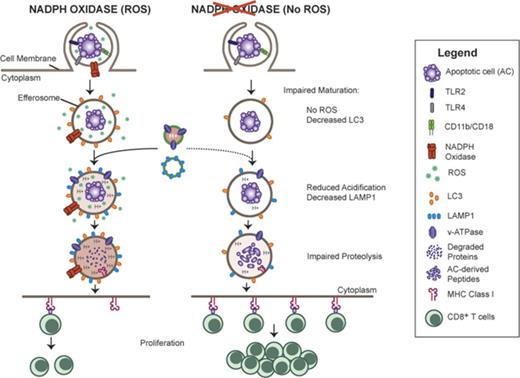
Key Points
Efferocytosis of ACs by inflammatory macrophages activates NADPH oxidase in a CD11b-TLR2/4-MyD88–dependent manner.
ROS generated positively regulate acidification and proteolysis in efferosomes, and limit cross-presentation of AC-associated antigens.
Abstract
The phagocyte reduced NAD phosphate (NADPH) oxidase generates superoxide, the precursor to reactive oxygen species (ROS) that has both antimicrobial and immunoregulatory functions. Inactivating mutations in NADPH oxidase alleles cause chronic granulomatous disease (CGD), characterized by enhanced susceptibility to life-threatening microbial infections and inflammatory disorders; hypomorphic NADPH oxidase alleles are associated with autoimmunity. Impaired apoptotic cell (AC) clearance is implicated as an important contributing factor in chronic inflammation and autoimmunity, but the role of NADPH oxidase–derived ROS in this process is incompletely understood. Here, we demonstrate that phagocytosis of AC (efferocytosis) potently activated NADPH oxidase in mouse peritoneal exudate macrophages (PEMs). ROS generation was dependent on macrophage CD11b, Toll-like receptor 2 (TLR2), TLR4, and myeloid differentiation primary response 88 (MyD88), and was also regulated by phosphatidylinositol 3-phosphate binding to the p40phox oxidase subunit. Maturation of efferosomes containing apoptotic neutrophils was significantly delayed in CGD PEMs, including acidification and acquisition of proteolytic activity, and was associated with slower digestion of apoptotic neutrophil proteins. Treatment of wild-type macrophages with the vacuolar-type H+ ATPase inhibitor bafilomycin also delayed proteolysis within efferosomes, showing that luminal acidification was essential for efficient digestion of efferosome proteins. Finally, cross-presentation of AC-associated antigens by CGD PEMs to CD8 T cells was increased. These studies unravel a key role for the NADPH oxidase in the disposal of ACs by inflammatory macrophages. The oxidants generated promote efferosome maturation and acidification that facilitate the degradation of ingested ACs.
Introduction
The reduced NAD phosphate (NADPH) oxidase is a multisubunit superoxide-generating enzyme that not only mediates oxidative killing of microbes but also is increasingly recognized for impacting other myeloid cell functions via still incompletely understood effects of derivative oxidants.1,2 Upon ligation of inflammatory receptors, the NADPH oxidase complex is rapidly assembled by translocation of cytosolic regulatory subunits to plasma or phagosomal membranes containing flavocytochrome b to generate superoxide (O2−) by the transfer of NADPH-derived electrons to molecular oxygen. The highly reactive O2− undergoes spontaneous or enzymatic conversion to other reactive oxygen species (ROS) important for microbial killing. Inactivating mutations in NADPH oxidase subunit alleles with loss of O2− production results in chronic granulomatous disease (CGD), an immunodeficiency characterized by susceptibility to life-threatening infections with opportunistic bacterial and fungal pathogens.3 CGD patients also exhibit inflammatory disorders including granulomas in the gastrointestinal and genitourinary tracts, and mucocutaneous skin lesions resembling discoid lupus. Moreover, recent genetic studies link hypomorphic NADPH oxidase subunit alleles with lupus, inflammatory bowel disease, and rheumatoid arthritis.4-12 However, mechanistic insights into the role of NADPH oxidase–derived oxidants in attenuating inflammation and the potential for autoimmunity are still limited.
Removal of exudate neutrophils at inflamed sites by inflammatory macrophages is important for resolution of inflammation. Prompt phagocytosis of apoptotic neutrophils, a process termed efferocytosis, ensures removal prior to their necrosis and culminates in the digestion of apoptotic cargo.13 Numerous studies have characterized the presentation of “eat me signals” on the surface of apoptotic cells (ACs), such as exposure of phosphatidylserine, and macrophage receptors that recognize ACs and trigger tethering and ingestion.13-15 Genetic mutations or environmental factors that impair AC recognition and/or uptake are associated with aberrant inflammation, immune dysfunction, and systemic autoimmunity.13,15 However, much less is known about the pathways that mediate subsequent disposal of ingested ACs.
Surprisingly, despite its relevance to resolution of inflammation, there is very little information on whether macrophage receptors engaged during efferocytosis activate the NADPH oxidase and whether this impacts digestion of ACs and processing of associated antigens. NADPH oxidase–derived ROS are implicated in regulating at least 2 processes that could impact efficient degradation of cell corpses. First, NADPH oxidase influences phagosome luminal chemistries through redox-mediated inactivation of cysteine cathepsin proteases16,17 and, in dendritic cells (DCs), luminal pH.18,19 Indeed, phagosomal proteolysis of proteins delivered on IgG-opsonized beads was increased in CGD macrophages and DCs, leading to reduced antigen presentation.16-18 Second, coupling of microtubule-associated protein light chain 3 (LC3) to the outer phagosome membrane, a form of noncanonical autophagy often referred to as “LC3-associated phagocytosis” (LAP), is stimulated by the NADPH oxidase.20-22 LAP is triggered during phagocytosis in response to Toll-like receptor (TLR) ligands and other microbial pathogen-associated molecular patterns, immunoglobulin G (IgG)-coated particles, and ACs.20,22-24 Depending on the cargo and cell type, ATG proteins and LAP accelerate or delay phagosome maturation to the acidic phagolysosome.21,23-27 Similarly, NADPH oxidase activity has a variable effect on modulating phagosome maturation.16-18,25 However, whether the NADPH oxidase regulates phagosomal proteolysis, maturation, and/or LC3 recruitment following efferocytosis by inflammatory macrophages remains undefined.
Here, we demonstrate that ingestion of apoptotic neutrophils by mouse peritoneal exudate macrophages (PEMs) activated the NADPH oxidase in a CD11b-TLR2/TLR4-myeloid differentiation primary response 88 (MyD88)–dependent manner. In turn, the oxidants generated promoted efferosome maturation, including LC3 acquisition, acidification, lysosomal fusion, and proteolytic degradation of ingested ACs. These events were significantly delayed in CGD-macrophage efferosomes compared with wild-type (WT) macrophages. Proteolysis in WT-macrophage efferosomes was inhibited by the vacuolar-type H+ ATPase (V-ATPase) inhibitor bafilomycin, demonstrating that acidification is important for digestion of AC-associated proteins. Moreover, cross-presentation of AC-associated antigens was enhanced in CGD macrophages. Our studies identify key molecular pathways in inflammatory macrophages that mediate NADPH oxidase activation in response to apoptotic neutrophils, leading to their efficient degradation following ingestion.
Materials and methods
Mice
CD44−/−,28 Itgam−/−,29 OT-1 mice expressing the ovalbumin (OVA)257–264/Kb–specific T-cell receptor,30 Tlr4−/− mice (C57BL/10ScN)31 and C57Bl/6J WT mice were purchased from The Jackson Laboratory. Mice with inactivation of X-linked Cybb (CGD mice)32 in C57Bl/6J or B6.SJL-PtrcaPep3b/BoyJ33 backgrounds were bred in-house. MyD88−/−34 and TLR2−/−35 mice were obtained from L.G.S. All mice were maintained in specific-pathogen-free conditions and used between 8 to 16 weeks of age. The institutional animal care and use committees at Washington University in St. Louis (WUSM) and at Indiana University Medical School (IUSM) approved all animal experiments.
PEM isolation
Mice were injected with 5 mM sodium periodate,36 sacrificed after 72 to 96 hours, and peritoneal cavities lavaged with phosphate-buffered saline containing 2 mM EDTA. Total lavage cells were plated in tissue-culture plates or chamber slides in Iscove modified Dulbecco medium with 10% heat-inactivated fetal bovine serum for 2 hours, nonadherent cells removed, and adherent macrophages cultured overnight before use in experiments.
Apoptotic cells
Human peripheral venous blood was obtained from healthy donors with informed consent, as approved by the institutional review boards at WUSM and IUSM. Neutrophils were purified using Polymorphprep (CosmoBio USA) as per the manufacturer’s instructions, and spontaneous apoptosis induced by culturing for 20 to 24 hours in RPMI-1640 containing 5% heat-inactivated fetal bovine serum at 5 × 106 cells per mL.36 In some experiments, human apoptotic neutrophils (hANs) were opsonized with exogenous complement by incubating at 37°C for an additional 30 minutes with RPMI containing 10% pooled human serum. Apoptosis in Jurkat T cells or in murine neutrophils was induced using UV irradiation. Apoptosis was assessed using Annexin V (BD Biosciences) and 7-amino-actinomycin D (7-ADD; Thermo Fisher) (supplemental Figure 1A, available on the Blood Web site). Assays used preparations with 60% to 80% early apoptotic (Annexin V+, 7-ADD−) and <10% late apoptotic or necrotic cells (Annexin V+, 7-ADD+).
NADPH oxidase and myeloperoxidase assays
NADPH oxidase activity was monitored by lucigenin or luminol chemiluminescence in a Spectramax L Luminometer (Molecular Devices) or by nitroblue tetrazolium (NBT).20,37,38 Neutrophil myeloperoxidase (MPO) following efferocytosis was determined by 3,3′-diaminobenzidine (DAB) staining36 or by western blotting.
Phagocytosis assays
PEMs seeded on 8-well glass chamber slides were stimulated with either hANs or IgG-coated latex beads for up to 30 minutes at 37°C. Slides were then washed 3 times with sterile phosphate-buffered saline, which removed any uningested hANs or IgG beads, and fixed using 4% paraformaldehyde prior to confocal microscopy or cultured for various time periods to monitor the fate of the ingested material. Phagosome acidification was monitored by adding Lysotracker Green (Thermo Fisher Scientific) for 5 minutes just prior to fixing at the indicated times. To assess proteolysis in phagosomes, IgG beads or hANs were labeled with a self-quenched BODIPY dye conjugated to bovine serum albumin (DQ-BSA green; Thermo Fisher Scientific).39 For pharmacological manipulation of ROS, PEMs were preincubated for 30 minutes with a ROS-inducing compound 100 μM tert-butyl hydrogen peroxide (TBHP) or 1 mM Tiron, a ROS scavenger, followed by feeding with ACs in the presence of TBPH or Tiron. In some experiments, V-ATPase activity was inhibited by preincubation with 1 μM bafilomycin A1 for 30 minutes at 37°C before the addition of phagocytic particles. Immunofluorescent staining for other markers of phagosome maturation was conducted using established methods.20,25,39,40
In vitro antigen-presentation assays
Thymocytes from WT mice were osmotically loaded with lipopolysaccharide-free OVA (Invivogen) as described,41 irradiated at 1400 cGy, and then incubated at 37°C for 3 hours to induce apoptosis. Apoptotic thymocytes (ATs) were fed to WT and CGD PEMs at different doses in the presence of carboxyfluorescein succinimidyl ester (CFSE)-labeled CD8+ OT1 cells.42 OT1 proliferation was assessed as CFSE dilution by flow cytometry after 72 hours. Data were collected on FACSCanto (BD Biosciences) or Cytek-modified FACScan (BD Biosciences and Cytek Development) instruments and analyzed with FlowJo (Tree Star).
Statistics
Statistical analyses used GraphPad Prism 6.0 (GraphPad Software Inc). Specific tests are noted in “Results” and/or in figure legends. A P value <.05 was considered statistically significant. If results represent data from multiple experiments, mean values ± standard deviation (SD) are shown (see supplemental Methods for additional details on generation of ACs, NADPH oxidase and myeloperoxidase assays, confocal microscopy, and cytokine and PGE-2 measurements).
Results
Efferocytosis activates NADPH oxidase in PEMs
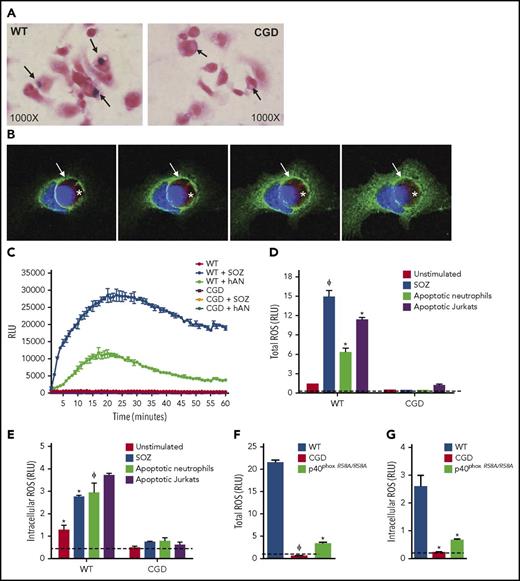
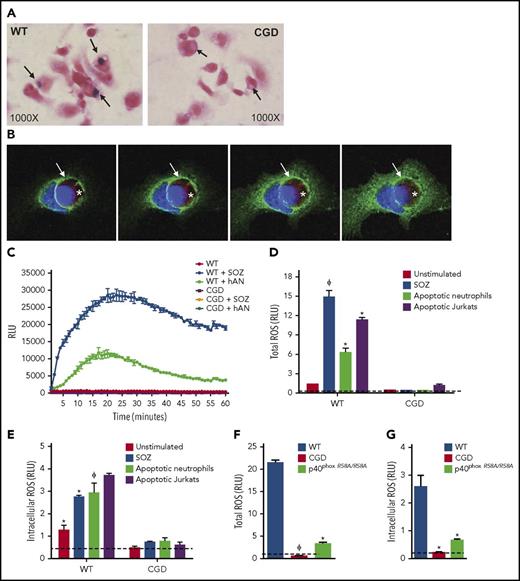
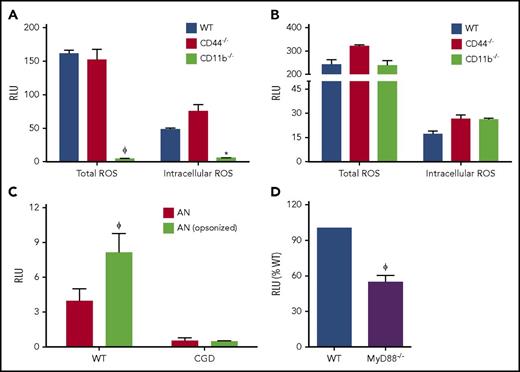
In our initial studies, we examined whether the NADPH oxidase is activated during efferocytosis of hANs by mouse PEMs. hANs were rapidly phagocytosed with similar efficiency by WT and CGD PEMs (supplemental Figure 1B). WT PEMs ingesting hANs in the presence of NBT accumulated purple formazan deposits within efferosomes indicative of ROS production, which were absent in CGD efferosomes (Figure 1A). Immunofluorescent staining revealed accumulation of macrophage gp91phox, a membrane-bound oxidase subunit, on WT efferosomes (Figure 1B). In this experiment, we used hANs prepared from a patient with X-linked CGD lacking gp91phox. Figure 1C shows kinetic profiles of ROS production by WT and CGD PEMs measured as lucigenin-elicited chemiluminescence after addition of hANs or serum-opsonized zymosan (SOZ). Both agonists generated robust ROS production in WT but not CGD PEMs. In addition to hANs, other ACs, such as apoptotic Jurkat cells and apoptotic murine neutrophils, also induced total and intracellular ROS levels comparable to those elicited by SOZ, a potent agonist of NADPH oxidase in macrophages37 (Figure 1D-E; supplemental Figure 1C-D). We also investigated a role for the p40phox subunit of the NADPH oxidase. p40phox binding to phosphoinositide 3 phosphate (PI(3)P) on phagosome membranes regulates phagosomal NADPH oxidase activity in mouse and human neutrophils,43,44 and in PEMs, stimulates NADPH oxidase activation both on the plasma membrane and on phagosomes.37 PEMs from p40phoxR58A/R58A mice harboring a nonfunctional PI(3)P-binding domain had significant defects in both total and intracellular ROS production during efferocytosis (Figure 1, panels F and G, respectively). This result indicates that the PI(3)P-p40phox axis upregulated efferocytosis-induced NADPH oxidase activity. Overall, our studies demonstrate that AC binding and ingestion strongly activated NADPH oxidase in mouse PEMs.
Efferocytosis leads to NADPH oxidase activation and oxidant production in mouse peritoneal macrophages. (A) WT and CGD PEMs plated on chamber slides were fed hANs at 1:5 (PEMs to hANs) in the presence of NBT for 30 minutes. Black arrows point to efferosomes. NBT oxidation by ROS leads to purple formazan deposits, observed in WT PEM efferosomes, whereas formazan-negative cytoplasmic inclusions can be observed in CGD PEMs. Images were acquired using 100× oil lens. (B) hANs were labeled with Cell Tracker Red (denoted with a white asterisk) from an X-CGD patient were fed to WT mouse PEMs for 20 minutes. gp91phox recruitment (green ring; white arrow) and 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining (blue) was assessed by confocal microscopy. Images were acquired in a confocal microscope using 100× oil lens. Images from left to right denote confocal images taken at different planes (top to bottom). (C) PEMs from WT and CGD mice were stimulated with hANs, serum-opsonized zymosan (SOZ), and ROS production monitored using lucigenin-elicited chemiluminescence. Response kinetics are shown in panel C as mean ± SD from 1 of 5 experiments. Total integrated relative light units per second (RLU) over 1 hour from duplicate wells of WT or CGD PEMs after stimulation with hANs, apoptotic Jurkat cells, or SOZ from 3 independent experiments are shown for (D) total ROS and (E) intracellular ROS. (F) Total and (G) intracellular ROS production in WT and p40phoxR58A/R58A PEMs after addition of hANs. Statistical differences between groups were calculated using 2-way analysis of variance (ANOVA) with Bonferroni posttest correction. *P < .05; ϕP < .01.
Efferocytosis leads to NADPH oxidase activation and oxidant production in mouse peritoneal macrophages. (A) WT and CGD PEMs plated on chamber slides were fed hANs at 1:5 (PEMs to hANs) in the presence of NBT for 30 minutes. Black arrows point to efferosomes. NBT oxidation by ROS leads to purple formazan deposits, observed in WT PEM efferosomes, whereas formazan-negative cytoplasmic inclusions can be observed in CGD PEMs. Images were acquired using 100× oil lens. (B) hANs were labeled with Cell Tracker Red (denoted with a white asterisk) from an X-CGD patient were fed to WT mouse PEMs for 20 minutes. gp91phox recruitment (green ring; white arrow) and 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining (blue) was assessed by confocal microscopy. Images were acquired in a confocal microscope using 100× oil lens. Images from left to right denote confocal images taken at different planes (top to bottom). (C) PEMs from WT and CGD mice were stimulated with hANs, serum-opsonized zymosan (SOZ), and ROS production monitored using lucigenin-elicited chemiluminescence. Response kinetics are shown in panel C as mean ± SD from 1 of 5 experiments. Total integrated relative light units per second (RLU) over 1 hour from duplicate wells of WT or CGD PEMs after stimulation with hANs, apoptotic Jurkat cells, or SOZ from 3 independent experiments are shown for (D) total ROS and (E) intracellular ROS. (F) Total and (G) intracellular ROS production in WT and p40phoxR58A/R58A PEMs after addition of hANs. Statistical differences between groups were calculated using 2-way analysis of variance (ANOVA) with Bonferroni posttest correction. *P < .05; ϕP < .01.
Efferocytosis activates NADPH oxidase in a CD11b-TLR2/4-MyD88–dependent manner
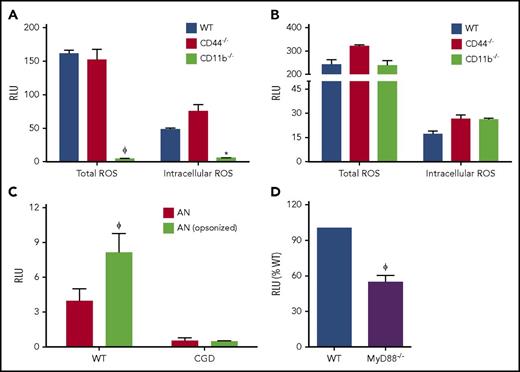
Various receptors, including scavenger receptors, CD44, Tim4, CD36, and complement receptors (CR3, CR4), mediate AC uptake by macrophages.15 However, the specific receptors and downstream signals triggering NADPH oxidase activation during macrophage efferocytosis are uncharacterized. CR3 (CD11b/CD18) is a promiscuous receptor that binds to many ligands,45 including iC3b-opsonized ACs,46,47 and is known to strongly activate the oxidative burst in neutrophils.48 The hyaluronan-binding receptor CD44 is also implicated in macrophage NADPH oxidase activation.49 We determined whether CR3 and CD44 regulated oxidase activation during efferocytosis by PEMs. The relative uptake of hANs was similar between CD44−/−, D11b−/−, and WT PEMs (supplemental Figure 2A). Deletion of CR3 resulted in a substantial reduction in total and intracellular ROS production in efferocytosing macrophages, whereas CD44 was dispensable for NADPH oxidase activation (Figure 2A). CD11b−/− PEMs were not intrinsically deficient in NADPH oxidase activity as ROS responses to unopsonized zymosan were unaffected (Figure 2B). Because prior studies demonstrated that iC3b opsonization of AC increases their uptake,46,47 we examined the relationship between iC3b on apoptotic neutrophils and NADPH oxidase activation during efferocytosis. Neutrophils, other myeloid cells, and lymphocytes were recently shown to have endogenous intracellular C3 stores.50 Interestingly, we observed that hANs undergoing apoptosis accumulated human iC3b on their surface even in the absence of exogenous human serum (supplemental Figure 2B). Furthermore, opsonization of hANs with exogenous iC3b concomitantly induced higher uptake and a greater fraction of NBT+ efferosomes in WT PEMs (supplemental Figure 2C) along with a significant increase in total NADPH oxidase–derived ROS (Figure 2C). Thus, macrophage CR3 plays an important role in activating the NADPH oxidase during efferocytosis, at least in part via iC3b on the surface of apoptotic neutrophils.
Activation of NADPH oxidase during efferocytosis in a CD11b-MyD88–dependent manner. PEMs from WT, CD11b−/−, and CD44−/− mice were either stimulated with (A) hANs or (B) zymosan, and ROS production measured as lucigenin-elicited chemiluminescence. (C) WT PEMs were stimulated with hANs or hANs opsonized with pooled human serum and ROS detected using lucigenin. (D) PEMs from WT and Myd88−/− mice were stimulated with hANs. For each graph, total integrated responses measured as relative light units/s (RLU) recorded over 1 hour are shown. Data from 1 of 3 experiments (duplicate wells) are shown as mean ± SD. Statistical differences between groups were calculated using 2-way ANOVA with Bonferroni posttest correction. *P < .05; ϕP < .01.
Activation of NADPH oxidase during efferocytosis in a CD11b-MyD88–dependent manner. PEMs from WT, CD11b−/−, and CD44−/− mice were either stimulated with (A) hANs or (B) zymosan, and ROS production measured as lucigenin-elicited chemiluminescence. (C) WT PEMs were stimulated with hANs or hANs opsonized with pooled human serum and ROS detected using lucigenin. (D) PEMs from WT and Myd88−/− mice were stimulated with hANs. For each graph, total integrated responses measured as relative light units/s (RLU) recorded over 1 hour are shown. Data from 1 of 3 experiments (duplicate wells) are shown as mean ± SD. Statistical differences between groups were calculated using 2-way ANOVA with Bonferroni posttest correction. *P < .05; ϕP < .01.
Dying cells release cellular DNA, nucleotides, hyaluronan fragments, uric acid crystals, and other products into the extracellular milieu that are detected by host receptors, including TLR2 and TLR4.51 We determined whether these TLRs acted as coreceptors and, along with the downstream MyD88 adaptor protein, positively influenced macrophage efferocytosis and ROS production. MyD88−/−, TLR2−/−, and TLR4−/− PEMs each ingested a similar fraction of hANs as WT PEMs (supplemental Figure 2A) but displayed significantly reduced NADPH oxidase activity during efferocytosis (Figure 2D; supplemental Figure 2D). Thus, the NADPH oxidase was also activated in a TLR2/4-MyD88–dependent manner during efferocytosis by inflammatory macrophages.
NADPH oxidase promotes degradation of AC proteins by PEMs
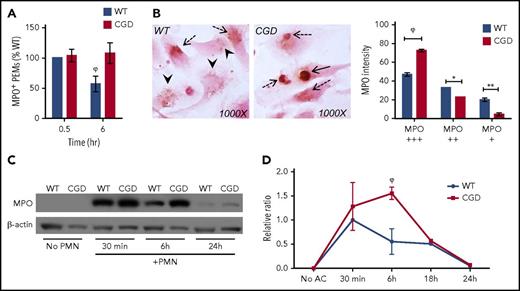
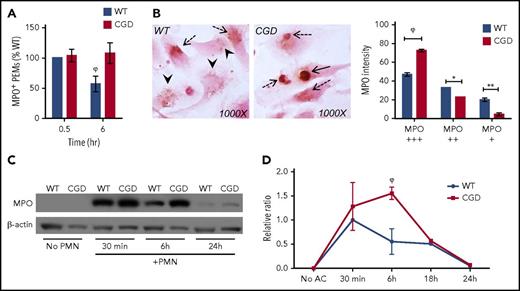
We tracked the fate of ingested hANs in WT and CGD macrophages to determine the impact of efferocytosis-induced NADPH oxidase activation on their degradation. PEMs were pulse-fed hANs for 30 minutes and incubated for an additional 6 hours. Cells were then stained for MPO, which is highly expressed in neutrophils, using DAB histochemistry. At each time point, total numbers of MPO+ (DAB+) PEMs were enumerated (Figure 3A), and the intensity of staining within individual DAB+ efferosomes scored (Figure 3B). Although WT and CGD PEMs ingested similar numbers of hANs during the 30-minute pulse (supplemental Figure 1A), a significantly greater fraction of CGD PEMs efferosomes after a 6-hour chase remained MPO+ compared with WT (Figure 3A). Moreover, MPO+ efferosomes remaining in WT PEMs after 6 hours had significantly less intense DAB staining than CGD PEMs containing MPO+ efferosomes (Figure 3B). Finally, western blot analysis for MPO levels within PEMs fed hANs showed significantly greater amounts of residual MPO within CGD PEMs after 6 hours compared with WT PEMs, despite similar initial ingestion of AC (supplemental Figure 1B; Figure 3C-D). Delayed degradation of hAN proteins was not limited to MPO, as significant delays were also observed for degradation of neutrophil elastase (supplemental Figure 3). Collectively, these results showed that impaired and delayed digestion of hANs occurs in the absence of phagosomal ROS and suggest that the NADPH oxidase is required to efficiently process ingested ACs in efferocytosing macrophages.
NADPH oxidase activation promotes digestion of neutrophil MPO within efferosomes. WT and CGD PEMs were pulse-fed hANs for 30 minutes, uningested hANs removed, and degradation of MPO determined in compared 6 hours by DAB histochemistry. (A) WT and CGD PEMss ingested similar numbers of hANs (30 minutes). Six hours postingestion CGD PEMs had significantly higher numbers of MPO+ macrophages compared with WT. (B) Averaged data from 2 to 4 experiments are shown. To compare relative digestion of ingested hANs, we determined the relative intensity of MPO staining in WT and CGD PEMs. Images were acquired using a 100× oil lens. Intensity of MPO staining was scored as “+++” (solid black arrows/strongly positive), “++” (dotted black arrows/intermediate intensity), or “+” (arrowheads/weakly positive) for each MPO+ efferosome at 6 hours and the percentage of distribution shown for each genotype. Results from 1 of 3 independent experiments are shown as mean ± SD. For panels A-C, at least 200 PEMs were scored for each genotype, per time point, and statistical differences between groups calculated using 2-way ANOVA with Bonferroni posttest correction. *P < .05; **P < .01; ϕP < .001. (C) PEMs were incubated with hANs for 30 minutes and chased for 6 or 24 hours. Lysates at the end of each time point were analyzed by western blot for MPO along with β-actin (loading control). Representative data from 1 of 5 independent experiments are shown. (D) Relative MPO band intensities normalized to β-actin were determined by ImageJ from samples from 3 independent experiments. Statistical differences between each time point calculated using the Student t test. ϕP < .001.
NADPH oxidase activation promotes digestion of neutrophil MPO within efferosomes. WT and CGD PEMs were pulse-fed hANs for 30 minutes, uningested hANs removed, and degradation of MPO determined in compared 6 hours by DAB histochemistry. (A) WT and CGD PEMss ingested similar numbers of hANs (30 minutes). Six hours postingestion CGD PEMs had significantly higher numbers of MPO+ macrophages compared with WT. (B) Averaged data from 2 to 4 experiments are shown. To compare relative digestion of ingested hANs, we determined the relative intensity of MPO staining in WT and CGD PEMs. Images were acquired using a 100× oil lens. Intensity of MPO staining was scored as “+++” (solid black arrows/strongly positive), “++” (dotted black arrows/intermediate intensity), or “+” (arrowheads/weakly positive) for each MPO+ efferosome at 6 hours and the percentage of distribution shown for each genotype. Results from 1 of 3 independent experiments are shown as mean ± SD. For panels A-C, at least 200 PEMs were scored for each genotype, per time point, and statistical differences between groups calculated using 2-way ANOVA with Bonferroni posttest correction. *P < .05; **P < .01; ϕP < .001. (C) PEMs were incubated with hANs for 30 minutes and chased for 6 or 24 hours. Lysates at the end of each time point were analyzed by western blot for MPO along with β-actin (loading control). Representative data from 1 of 5 independent experiments are shown. (D) Relative MPO band intensities normalized to β-actin were determined by ImageJ from samples from 3 independent experiments. Statistical differences between each time point calculated using the Student t test. ϕP < .001.
NADPH oxidase promotes maturation, acidification, and proteolytic activity within PEM efferosomes
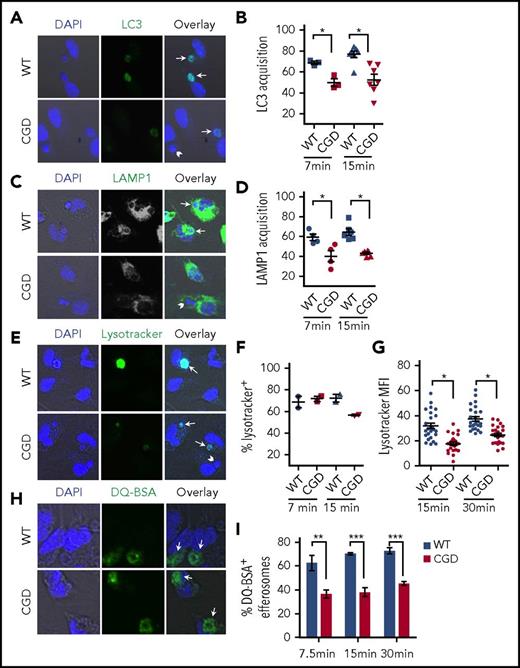
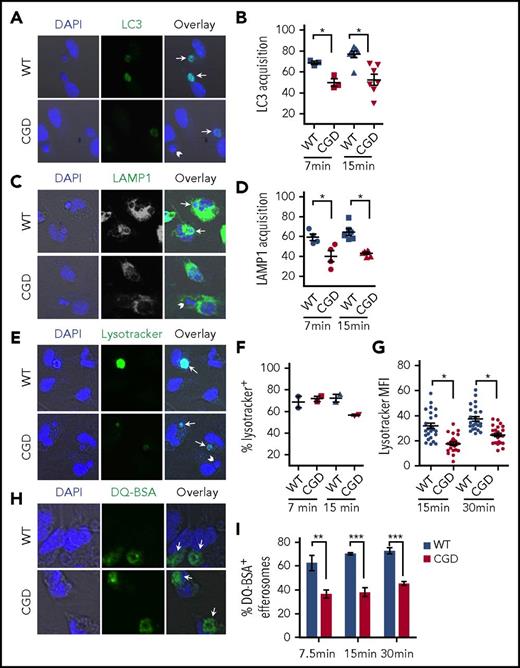
The slower degradation of hAN MPO by CGD PEMs could reflect delayed phagosome maturation, a series of sequential fusion and fission events with endosomes and lysosomes that remodel the nascent phagosome to form the mature acidic phagolysosome with a high degradative capacity.52 We therefore compared kinetics of different maturation events after ingestion of hANs by WT and CGD PEMs. Acquisition of LC3 and the late endosome/lysosome marker LAMP-1 on efferosomes was significantly delayed in CGD PEMs (Figure 4A-D). The increasingly acidic environment in the macrophage phagosome, which is insensitive to the electrogenic effects of the NADPH oxidase,17,19,53 is mediated by V-ATPases delivered by late endosome tubular structures.54,55 We monitored efferosome acidification using Lysotracker Green (Figure 4E), a fluorescent weak base that accumulates in the phagosome after protonation, thus serving as an indirect measure of pH.56 The majority of efferosomes were Lysotracker-positive in WT and CGD PEMs by 7 minutes (Figure 4F). However, Lysotracker fluorescence was noticeably brighter in WT efferosomes compared with CGD (Figure 4E), and Lysotracker fluorescence intensity quantitated for individual WT efferosomes was significantly higher compared with CGD PEMs at 7.5 (not shown), 15, and 30 minutes (Figure 4G). These results suggest reduced or slower acidification of efferosomes in the absence of NADPH oxidase. Finally, to assess the proteolytic environment, we monitored the cleavage of the protein DQ-BSA coated onto hANs. DQ-BSA is a model fluorogenic substrate that when cleaved produces a bright fluorescence, which was scored at various time points after AC feeding. The fraction of efferosomes with cleaved DQ-BSA was significantly lower in CGD PEMs at 7.5, 15, and 30 minutes compared with WT PEMs (Figure 4H-I), indicating impaired proteolysis of efferosomal cargo. Taken together, these results demonstrated that efferosomes in NADPH oxidase–deficient macrophages have delayed maturation and impaired functional capacities following ingestion of apoptotic neutrophils.
NADPH oxidase deficiency is associated with delayed maturation of efferosomes. hANs were incubated with WT or CGD PEMs for 7, 15, or 30 minutes as indicated, and efferosome maturation assessed using confocal microscopy–based assays. Images were acquired in a confocal microscope using a 40× oil lens. Representative confocal images shown were taken at 7 minutes, with marker-positive efferosomes indicated by arrows and marker-negative indicated by arrowheads. The percentage of efferosomes positive for the specified markers were analyzed for each genotype (WT = blue; CGD = red) and shown in the panels to the right of each confocal image. (A-B) LC3 acquisition or (C-D) Lamp1 acquisition at 7.5 and 15 minutes. (E-G) Lysotracker Green was added to PEMs for 5 minutes prior to the indicated time points to compare relative acidification of efferosomes. Panel F shows relative fraction of Lysotracker-positive efferosomes in WT and CGD PEMs. (G) MFI in individual Lysotracker-positive WT and CGD efferosomes PEMs 15 and 30 minutes post hANs feeding in 1 representative experiment. MFI was determined using ImageJ. (H-I) To compare proteolysis rates, hANs were coated with DQ-BSA and subsequently fed to PEMs. (I) The fraction of brightly green fluorescent efferosomes in WT and CGD at 7.5, 15, and 30 minutes postfeeding, indicating cleavage of DQ-BSA and unquenching of the BOPIDY dye, whose fluorescence is insensitive to pH over a wide range. Cumulatively, 20 to 50 efferosomes in each of >3 experiments were analyzed for each time point studied for each treatment condition for WT and CGD. Statistical differences between genotypes were measured for each time point using the Student t test (mean ± SD). *P < .05; **P < .01; ***P < .001.
NADPH oxidase deficiency is associated with delayed maturation of efferosomes. hANs were incubated with WT or CGD PEMs for 7, 15, or 30 minutes as indicated, and efferosome maturation assessed using confocal microscopy–based assays. Images were acquired in a confocal microscope using a 40× oil lens. Representative confocal images shown were taken at 7 minutes, with marker-positive efferosomes indicated by arrows and marker-negative indicated by arrowheads. The percentage of efferosomes positive for the specified markers were analyzed for each genotype (WT = blue; CGD = red) and shown in the panels to the right of each confocal image. (A-B) LC3 acquisition or (C-D) Lamp1 acquisition at 7.5 and 15 minutes. (E-G) Lysotracker Green was added to PEMs for 5 minutes prior to the indicated time points to compare relative acidification of efferosomes. Panel F shows relative fraction of Lysotracker-positive efferosomes in WT and CGD PEMs. (G) MFI in individual Lysotracker-positive WT and CGD efferosomes PEMs 15 and 30 minutes post hANs feeding in 1 representative experiment. MFI was determined using ImageJ. (H-I) To compare proteolysis rates, hANs were coated with DQ-BSA and subsequently fed to PEMs. (I) The fraction of brightly green fluorescent efferosomes in WT and CGD at 7.5, 15, and 30 minutes postfeeding, indicating cleavage of DQ-BSA and unquenching of the BOPIDY dye, whose fluorescence is insensitive to pH over a wide range. Cumulatively, 20 to 50 efferosomes in each of >3 experiments were analyzed for each time point studied for each treatment condition for WT and CGD. Statistical differences between genotypes were measured for each time point using the Student t test (mean ± SD). *P < .05; **P < .01; ***P < .001.
ROS produced during efferocytosis regulate efferosome maturation and proteolysis
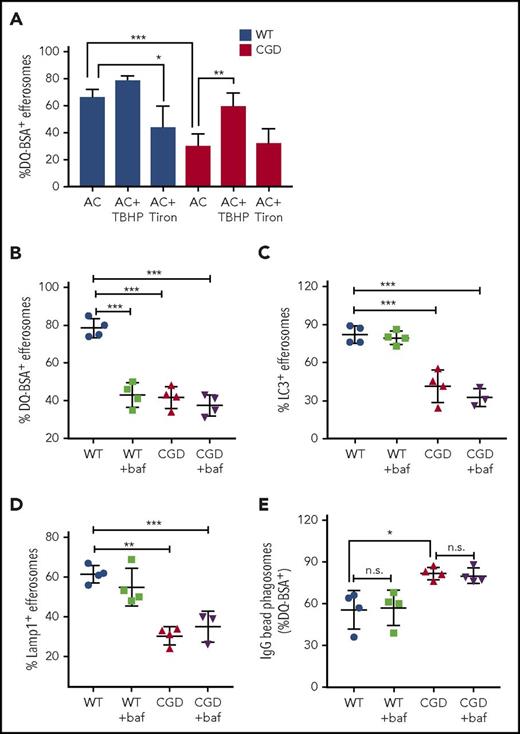
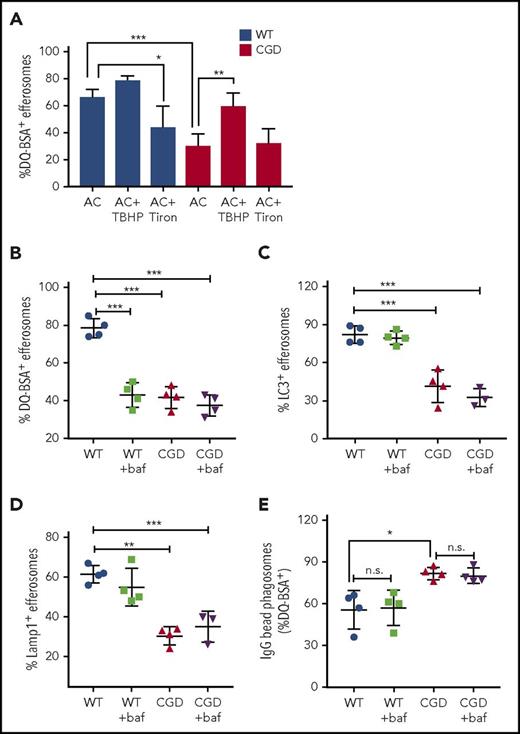
As another approach to establish that ROS generated by the NADPH oxidase during efferocytosis regulated efferosome maturation and proteolysis, we manipulated oxidant levels using the ROS generator TBHP or the ROS scavenger Tiron.57 TBHP treatment was sufficient to generate oxidants in CGD PEMs (supplemental Figure 4B). TBHP treatment of CGD PEMs significantly improved proteolysis within CGD efferosomes, as measured by DQ-BSA cleavage (Figure 5A). Acquisition of LC3 and Lamp1 on CGD efferosomes was also enhanced (supplemental Figure 4C-D). In contrast, the oxidant scavenger Tiron, which ablated hAN-induced ROS by WT PEMs (supplemental Figure 4A), reduced DQ-BSA processing and recruitment of LC3 and LAMP1 in WT but not CGD efferosomes (Figures 5A; supplemental Figure 4C-D). Thus, we conclude that ROS generated by NADPH oxidase during efferocytosis promotes efferosome maturation and the generation of a proteolytic environment in WT PEMs.
Oxidants produced during efferocytosis and efferosome acidification regulate efferosome maturation and proteolysis. (A) WT (black bars) and CGD (white bars) PEMs were preincubated (30 minutes) either with 100 μM TBHP (TB) or 1 mM Tiron. PEMs were then fed DQ-BSA–coated hANs for 15 minutes and the percentage of efferosomes showing DQ-BSA cleavage determined. PEMs were also pretreated with bafilomycin A1 for 30 minutes before feeding with hANs or DQ-BSA–coated hANs for 10 minutes and the fraction of efferosomes exhibiting (B) DQ-BSA-cleavage, (C) LC3 localization, and (D) Lamp1 recruitment was determined. Results from 4 independent experiments are shown as means ± SD. *P < .05; **P < .01, ***P < .001 by the Student t test. (E) Polystyrene beads coated with IgG and DQ-BSA were fed to PEMs at a ratio of 20:1 for 10 minutes. Some PEMs pretreated with 1 μM bafilomycin for 30 minutes before and during the addition of beads, as indicated. After imaging with confocal microscopy, the percentage of efferosomes containing cleaved DQ-BSA was calculated. Results from 4 experiments were shown. *P < .05 by Student t test.
Oxidants produced during efferocytosis and efferosome acidification regulate efferosome maturation and proteolysis. (A) WT (black bars) and CGD (white bars) PEMs were preincubated (30 minutes) either with 100 μM TBHP (TB) or 1 mM Tiron. PEMs were then fed DQ-BSA–coated hANs for 15 minutes and the percentage of efferosomes showing DQ-BSA cleavage determined. PEMs were also pretreated with bafilomycin A1 for 30 minutes before feeding with hANs or DQ-BSA–coated hANs for 10 minutes and the fraction of efferosomes exhibiting (B) DQ-BSA-cleavage, (C) LC3 localization, and (D) Lamp1 recruitment was determined. Results from 4 independent experiments are shown as means ± SD. *P < .05; **P < .01, ***P < .001 by the Student t test. (E) Polystyrene beads coated with IgG and DQ-BSA were fed to PEMs at a ratio of 20:1 for 10 minutes. Some PEMs pretreated with 1 μM bafilomycin for 30 minutes before and during the addition of beads, as indicated. After imaging with confocal microscopy, the percentage of efferosomes containing cleaved DQ-BSA was calculated. Results from 4 experiments were shown. *P < .05 by Student t test.
V-ATPase–dependent acidification regulates proteolysis in PEM efferosomes
We next examined whether acidification regulated proteolysis of AC-associated DQ-BSA. Luminal acidification enhances the activity of many lysosomal proteases and other enzymes that degrade phagosomal contents. We hypothesized that the delayed degradation of AC-associated proteins in CGD PEMs (Figures 3-4) was related to slower acidification. We inhibited acidification in PEMs ingesting DQ-BSA–coated hANs using the specific V-ATPase inhibitor bafilomycin.58-61 Although bafilomycin did not reduce the fraction of Lysotracker-positive efferosomes, the mean fluorescence intensity (MFI) of the Lysotracker signal was significantly reduced, particularly in WT efferosomes, consistent with reduced acidification (supplemental Figure 4E). Bafilomycin reduced the fraction of efferosomes in WT PEMs that contained cleaved DQ-BSA 10 minutes after hAN feeding to a level similar to CGD PEM efferosomes (Figure 5B). Bafilomycin did not further reduce the fraction of efferosomes with cleaved DQ-BSA in CGD PEMs. Notably, LC3 (Figure 5C) and Lamp1 recruitment (Figure 5D) were not affected in bafilomycin-treated PEMs, showing that inhibition of V-ATPase did not alter other measures of phagosome maturation. These results indicate that V-ATPase–dependent luminal acidification is a key regulator of proteolytic activity within inflammatory macrophage efferosomes and suggest that the impaired acidification of CGD efferosomes (Figure 4G) leads to slower degradation of AC-associated proteins.
NADPH oxidase inhibits maturation and proteolytic activity of IgG-bead phagosomes in PEMs
Interestingly, the delayed proteolysis within CGD PEM efferosomes contrasts with a study by Rybicka et al,17 who found that digestion of proteins attached to IgG-coated beads was more rapid in CGD bone marrow–derived macrophage (BMDM) phagosomes due to reduced oxidative inactivation of cysteine cathepsins. We performed studies to assess whether these disparate results reflected differences in the 2 types of macrophages. However, similar to Rybicka et al,17 we found that proteolytic cleavage of IgG-bead–associated DQ-BSA was enhanced in CGD PEMs compared with WT PEMs (Figure 5E). Moreover, addition of bafilomycin did not reduce cleavage of DQ-BSA on IgG beads (Figure 5E). This indicates that V-ATPase–dependent phagosome acidification was not required for efficient digestion of proteins attached to IgG-opsonized beads. These results demonstrate that NADPH oxidase activity can either positively or negatively regulate the degradative capacity of phagosomes, depending on the nature of phagosomal cargo.
NADPH oxidase regulates cross-presentation of AC-derived antigens by PEMs
Next, we examined the consequences of delayed AC digestion. Efferocytosis of hANs did not differentially induce proinflammatory cytokines interleukin-1β (IL-1β) and IL-6, and there was also no significant induction of IL-10 (Figure 6A). Moreover, although efferocytosis induced the production of the anti-inflammatory mediators TGF-β and PGE-2, these levels were similar for WT and CGD PEMs (Figure 6A).
CGD macrophages have increased cross-presentation of AC-derived antigens. (A) WT and CGD PEMs were plated in Permanox chamber slides, cultured overnight, and the next day stimulated with hANs for an additional 18 hours. Cell-free supernatants were analyzed by 32-cytokine multiplex cytokine arrays or enzyme linked immunosorbent assays (transforming growth factor β [TGF-β] and prostaglandin E2 [PGE-2]). Data from 1 of 2 experiments using 3 mice per genotype are shown as mean ± SD. Statistical differences were determined by 1-way ANOVA with the Tukey postcorrection test. **P < .01. (B-C) WT and CGD PEMs were incubated for 72 hours with either varying numbers of OVA-loaded ATs or soluble OVA in the presence of CFSE-labeled CD8 T cells derived from transgenic OT1 mice. At the end of 72 hours, proliferation of CD8 OT1 T cells was measured by flow cytometry as CFSE dilution, and the percentage of proliferated T cells determined for each stimulation condition and agonist concentration. Data (mean ± SD) from 1 of 3 representative experiments are shown. **P < .01 using the Student t test.
CGD macrophages have increased cross-presentation of AC-derived antigens. (A) WT and CGD PEMs were plated in Permanox chamber slides, cultured overnight, and the next day stimulated with hANs for an additional 18 hours. Cell-free supernatants were analyzed by 32-cytokine multiplex cytokine arrays or enzyme linked immunosorbent assays (transforming growth factor β [TGF-β] and prostaglandin E2 [PGE-2]). Data from 1 of 2 experiments using 3 mice per genotype are shown as mean ± SD. Statistical differences were determined by 1-way ANOVA with the Tukey postcorrection test. **P < .01. (B-C) WT and CGD PEMs were incubated for 72 hours with either varying numbers of OVA-loaded ATs or soluble OVA in the presence of CFSE-labeled CD8 T cells derived from transgenic OT1 mice. At the end of 72 hours, proliferation of CD8 OT1 T cells was measured by flow cytometry as CFSE dilution, and the percentage of proliferated T cells determined for each stimulation condition and agonist concentration. Data (mean ± SD) from 1 of 3 representative experiments are shown. **P < .01 using the Student t test.
We also investigated whether delayed proteolysis in CGD PEMs efferosomes (Figures 3-4) preserved AC-associated antigens and consequently increased cross-presentation compared with WT PEMs. We used OVA as a model antigen for these assays, and CD8 T cells derived from OT-1 mice that recognize the peptide OVA257-264 in the context of H2Kb-specific T-cell receptors. OVA-loaded ATs were fed to WT and CGD PEMs at different AT-to-PEMs ratios in the presence of CFSE-labeled OT1 CD8 T cells. Significantly higher proliferation of CD8 T cells in wells containing CGD PEMs (Figure 6B) indicated enhanced presentation of AC-associated OVA. In contrast, we did not observe any differences in the presentation of soluble OVA that was not associated with ACs (Figure 6C). Thus, our studies indicate that the lower proteolytic capacity of CGD efferosomes resulted in preservation of antigenic epitopes for presentation to CD8 T cells.
Discussion
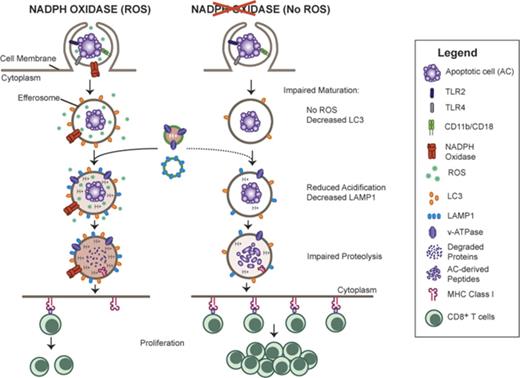
Prompt disposal of ACs is important to prevent aberrant inflammatory responses. Dysregulated AC clearance can accelerate the progression of autoimmune disorders, infectious diseases, and even influence the immunogenicity of dying tumor cells.15 Thus, a closer understanding of the molecular and cellular events to process and eventually dispose of ingested apoptotic cargo is essential. In this study, we demonstrate a direct role for NADPH oxidase–derived ROS in regulating the clearance of apoptotic neutrophils following their ingestion by inflammatory macrophages. ROS generated were essential for maturation of efferosomes, including acquisition of their acidic as well as proteolytic capacities. Lacking NADPH oxidase activity, CGD PEMs exhibited significant delays in efferosomal maturation and proteolytic breakdown of ingested ACs, leading to persistence of AC proteins within phagosomes and enhanced presentation of an AC-associated protein antigen.
Little was previously known about activation of the NADPH oxidase complex during efferocytosis. The majority of murine macrophage flavocytochrome b558, a membrane heterodimer of gp91phox and p22phox, resides in Rab11+ recycling endosomes, and traffics to phagosome membranes during phagocytosis.40 Here, we showed that AC ingestion swiftly induced activation of the NADPH oxidase, with localization of gp91phox on efferosome membranes. Maximal ROS generation required CR3, p40phox binding to PI(3)P as well as the TLR-MyD88–signaling axis. Furthermore, deposited complement on the AC surface significantly enhanced ROS generation. Prior studies demonstrated a link between iC3b opsonization of ACs and their uptake.46,47 Our work extends the importance of CR3 for disposal of ACs, revealing a role for CR3 in activating the NADPH oxidase to promote efficient macrophage degradation of ACs.
Regulation of phagosome maturation is complex and is increasingly recognized to have important differences that depend on the cargo and cell type.21,23-26 Here, we showed that recruitment of LC3 to CGD PEMs efferosomes was impaired compared with WT PEMs, extending prior observations showing that NADPH oxidase–derived ROS are upstream of LC3 acquisition by zymosan, IgG-bead, or TLR-bead macrophage phagosomes.20-22 Impaired LC3 recruitment in inflammatory macrophages was associated with delayed efferosome maturation and timely digestion of AC-associated proteins. Our studies did not undertake a comprehensive analysis of all AC-associated proteins. Although we demonstrate that degradation of MPO and elastase from apoptotic neutrophils was delayed in CGD PEMs, whether these observations extend to all AC-associated proteins or to apoptotic murine neutrophils needs to be determined.
We also established that NADPH oxidase–derived ROS and luminal acidification differentially regulate phagosomal proteolysis in a manner that depends upon the nature of ingested cargo. Most studies investigating proteolysis in phagosomes have used relatively simple cargo, for example, opsonized inert particles like latex beads or silica particles. ACs, however, present a complex substrate and their breakdown within efferosomes involves disposal of proteins, lipids, and nucleic acids. We found that ROS were essential for efficient proteolysis within efferosomes of inflammatory macrophages. In contrast, NADPH oxidase ROS delayed proteolysis in phagosomes containing IgG-opsonized latex beads consistent with studies of Rybicka et al in BMDMs.17 We additionally showed that pharmacologic inhibition of V-ATPase activity reduced proteolytic activity in efferosomes but not in IgG-bead phagosomes. Acidic conditions enhance the activity of many lysosomal proteases and other hydrolases important for digesting AC material, which could directly or indirectly impact efficient digestion of AC-associated proteins. Apart from the inherent complexity of the substrate itself, other factors might account for the differences between digestion of proteins on IgG-coated particles compared with ACs. In macrophages and DCs, IgG opsonization of beads is reported to enhance LAMP-1 recruitment and proteolysis following their phagocytosis compared with phagosomes harboring unopsonized beads.62
The relationship between phagosomal pH and NADPH oxidase ROS is complex and differs between macrophages, DCs, and neutrophils.19 Macrophage phagosomes are strongly acidified by the action of V-ATPases, and luminal pH in macrophages ingesting IgG beads or zymosan is insensitive to electrogenic and ionic effects of the NADPH oxidase.17,19,53 The reason for impaired acidification of CGD efferosomes is uncertain, but may reflect a role for LC3 on recruitment of V-ATPases.
Limiting proteolysis of phagosomal cargo is correlated with the ability to generate antigenic peptides for presentation by major histocompatibility complex proteins and activation of adaptive immune responses.63 Our studies indicate enhanced cross-presentation of AC-derived antigens by CGD PEMs (Figure 6), unlike Amigorena and colleagues who observed decreased cross-presentation by CGD DCs as a result of increased proteolysis of peptides delivered on IgG-coated beads.18,64 Although the mechanism by which ROS reduce proteolysis in DCs is controversial,16,18,64 DCs overall have reduced lysosomal proteases compared with macrophages, which helps to preserve antigen integrity.62,65,66 Our studies investigated cross-presentation of AC-derived antigens in inflammatory macrophages, which typically mount a superior oxidative burst, rapidly acidify phagosomes, and have high levels of lysosomal proteases compared with DCs.19,66 Inflammatory macrophages can migrate to lymph nodes, and present AC-derived ligands during inflammation.67,68 Enhanced presentation of antigens by NADPH oxidase–deficient PEMs might explain some of the inflammatory and autoimmune complications seen in CGD and in individuals with hypomorphic NADPH oxidase alleles.4-12
In our studies, delayed efferosome maturation in CGD-exudate macrophages was not associated with altered production of proinflammatory or anti-inflammatory mediators. In contrast, ingestion of ACs by macrophages deficient in components of the LAP pathway was associated with markedly increased production of proinflammatory cytokines.27,69 A possible explanation might be that the prior studies used the BMDM, a relatively naive macrophage, and efferosome maturation reported for BMDMs27 was much slower than for WT and CGD PEMs, which could affect mediator production. Furthermore, we studied aged neutrophils undergoing spontaneous apoptosis, unlike the other studies, where apoptosis in various cell lines was induced using chemical or UV-based methods.27,69
Finally, these studies provide novel mechanistic insights into how alterations in macrophage functional responses in CGD can potentially dysregulate inflammatory responses. Neutrophils undergo apoptosis both constitutively and in inflamed tissues, and defects in their subsequent clearance can influence resolution of inflammation and homeostasis. Neutrophilic inflammation is prominent in CGD,70 and hyperinflammatory responses are observed in stimuli of varying nature, for example, zymosan,71 sterile Aspergillusfumigatus hyphae,72 monosodium urate crystals, and other damage-associated molecular patterns.37,73 Apoptotic CGD neutrophils generate reduced levels of lysophosphatidylserine, a lysophospholipid that enhances macrophage efferocytosis, and relatively higher numbers of uningested apoptotic neutrophils were present during sterile inflammation in CGD mice.73,74 Increased numbers of exudate macrophages containing apoptotic polymorphonuclear neutrophils were also observed,36,71,73 which could reflect both increased neutrophil burden and slower degradation following their ingestion. Hence, defects in both the uptake and digestion of apoptotic polymorphonuclear neutrophils may contribute to aberrant inflammation and risk of autoimmunity in NADPH oxidase deficiency.63
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Tina McGrath for assistance with manuscript preparation.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute grants R01HL045635 (M.C.D.) and R01HL134896 (L.G.S.), Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (M.C.D., L.G.S.), and internal funding from the University of Louisville (J.B.). M.Y.Z. was supported by National Institutes of Health National Institute of Allergy and Infectious Diseases grant T32 AI060519 at the Indiana University School of Medicine.
Authorship
Contribution: J.B., J.H., M.Y.Z., and M.C.D. designed research, analyzed data, and wrote the manuscript; J.B., J.H., M.Y.Z., N.K.P., L.J.P.-Z., and I.M. performed experiments; and D.A.M. and L.G.S. provided additional input and key reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary C. Dinauer, Washington University School of Medicine in St. Louis, 660 S. Euclid Ave, PO Box 8208, St. Louis, MO 63110; e-mail: mdinauer@wustl.edu.
REFERENCES
Author notes
J.B., J.H., and M.Y.Z. contributed equally to this work.

![Figure 6. CGD macrophages have increased cross-presentation of AC-derived antigens. (A) WT and CGD PEMs were plated in Permanox chamber slides, cultured overnight, and the next day stimulated with hANs for an additional 18 hours. Cell-free supernatants were analyzed by 32-cytokine multiplex cytokine arrays or enzyme linked immunosorbent assays (transforming growth factor β [TGF-β] and prostaglandin E2 [PGE-2]). Data from 1 of 2 experiments using 3 mice per genotype are shown as mean ± SD. Statistical differences were determined by 1-way ANOVA with the Tukey postcorrection test. **P < .01. (B-C) WT and CGD PEMs were incubated for 72 hours with either varying numbers of OVA-loaded ATs or soluble OVA in the presence of CFSE-labeled CD8 T cells derived from transgenic OT1 mice. At the end of 72 hours, proliferation of CD8 OT1 T cells was measured by flow cytometry as CFSE dilution, and the percentage of proliferated T cells determined for each stimulation condition and agonist concentration. Data (mean ± SD) from 1 of 3 representative experiments are shown. **P < .01 using the Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/21/10.1182_blood-2017-09-809004/4/m_blood809004f6.jpeg?Expires=1767729511&Signature=H32J8DGMVMtTx7T326yA2DM-LshavXvCML9CfVefgiFfD4QqzcFkSdbqvnbGJknsAnZmQ3YKnxjLdKblcfmmzyn1iVKEaDCC69DCA5K05uATDhm70X3NBOOYKo~pAwU~fNloEPVc8DQAe0QJ~WnjjedLQKfZvWdTgx0TMPhR4PMlxLwHSu6AfEnibkHlFrjXn4QqoQdO6oOQfbUH5TQZOnCYwOSU8Q-naRyiCSbYvenbwNk5bMlqRbSFr36AgfyvdhwY7CNDNIdxjTDyhNEuGEjyZyNBUCf4AsXH8gjBv1tGHIrabTcqeQ35RG0bHf-jpwFDqMcKhNpJQ~lzhMfrEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. CGD macrophages have increased cross-presentation of AC-derived antigens. (A) WT and CGD PEMs were plated in Permanox chamber slides, cultured overnight, and the next day stimulated with hANs for an additional 18 hours. Cell-free supernatants were analyzed by 32-cytokine multiplex cytokine arrays or enzyme linked immunosorbent assays (transforming growth factor β [TGF-β] and prostaglandin E2 [PGE-2]). Data from 1 of 2 experiments using 3 mice per genotype are shown as mean ± SD. Statistical differences were determined by 1-way ANOVA with the Tukey postcorrection test. **P < .01. (B-C) WT and CGD PEMs were incubated for 72 hours with either varying numbers of OVA-loaded ATs or soluble OVA in the presence of CFSE-labeled CD8 T cells derived from transgenic OT1 mice. At the end of 72 hours, proliferation of CD8 OT1 T cells was measured by flow cytometry as CFSE dilution, and the percentage of proliferated T cells determined for each stimulation condition and agonist concentration. Data (mean ± SD) from 1 of 3 representative experiments are shown. **P < .01 using the Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/21/10.1182_blood-2017-09-809004/4/m_blood809004f6.jpeg?Expires=1767729512&Signature=m75VygNtNr5kUp6diRdEgI4OqV2b7Xl2NZPDUD4naj6NfzO~3fYzTmkC~82PgyvK8n22pLklgt16KGcRWkdJm7ncPop-kwxbNGN5T4oapo3-T74b6jLrya-2nvyCxA6kdAM3aoDyDmjNP24FgrAVDuZvdK1gR6iLWyR1-lPo852zzcXeGFY~0C6-tIqgbHZpeDONAHWGr1iGVPOTNmDaywPtzX~9VN56uJQVaqi7EHIgnmxWOoJsy4jStelxfyFoUBk8HwxWXB~cx0GQV4E3cpRa0PItThmZ4WceRrcRsbwH~Y~uEk3bRSiJvs1Wjq6e~WxREc0I9NyLNQqObjbhtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)